- RETIREMENT ANNOUNCEMENT
- HOME PAGE
- "MYCHART" the new patient portal
- BELMONT MEDICAL ASSOCIATES
- MOUNT AUBURN HOSPITAL
- EMERGENCIES
- PRACTICE PHILOSOPHY
- MY RESUME
- TELEMEDICINE CONSULTATION
- CONTACT ME
- LAB RESULTS
- ePRESCRIPTIONS
- eREFERRALS
- RECORD RELEASE
- MEDICAL SCRIBE
- PHYSICIAN ASSISTANT (PA)
- Medicare Annual Wellness Visit
- Case management/Social work
- Quality Care Measures
- Emergency closing notice
- FEEDBACK
- Talking to your doctor
- Choosing..... and losing a doctor
- INDEX A - Z
- ALLERGIC REACTIONS
- Alternative Medicine
- Alzheimer's Disease
- Bladder Problems
- Blood disorders
- Cancer Concerns
- GENETIC TESTING FOR HEREDITARY CANCER
- Chronic Obstructive Pulmonary Disease
- Controversial Concerns
- CPR : Learn and save a life
- CRP : Inflammatory marker
- Diabetes Management
- Dizziness, Vertigo,Tinnitus and Hearing Loss
- EXERCISE
- FEMALE HEALTH
-
GASTROINTESTINAL topics
- Appendicitis
- BRAT diet
- Celiac Disease or Sprue
- Crohn's Disease
- Gastroenterologists for Colon Cancer Screening
- Colonoscopy PREP
- Constipation
- Gluten sensitivity, but not celiac disease
- Heartburn and GERD
- Hemorrhoids and Anal fissure
- Irritable Bowel Syndrome (IBS)
- Inflammatory Bowel Disease
- NASH : Non Alcoholic Steato Hepatitis
- FEET PROBLEMS
- HEART RELATED topics
-
INFECTIOUS DISEASES
- Antibiotic Resistance
- Cat bites >
- Clostridia difficile infection - the "antibiotic associated germ"
- CORONA VIRUS
- Dengue Fever and Chikungunya Fever
- Food borne illnesses
- Shingles Vaccine
- Hepatitis B
- Hepatitis C
- Herpes
- Influenza
- Helicobacter pylori - the "ulcer germ"
- HIV Screening
- Lyme and other tick borne diseases
- Measles
- Meningitis
- MRSA (Staph infection)
- Norovirus
- Sexually Transmitted Diseases
- Shingles (Herpes Zoster)
- Sinusitis
- West Nile Virus
- Whooping Cough (Pertussis)
- Zika virus and pregnancy
- INSURANCE related topics
- KIDNEY STONES
- LEG CRAMPS
- LIBRARY for patients
- LIFE DECISIONS
- MALE HEALTH
- Medication/Drug side effects
- MEDICAL MARIJUANA
- MENTAL HEALTH
- Miscellaneous Articles
-
NUTRITION - EXERCISE - WEIGHT
- Cholesterol : New guidelines for treatment
- Advice to lower your cholesterol
- Cholesterol : Control
- Cholesterol : Raising your HDL Level
- Exercise
- Food : Making Smart Choices
- Food : Making Poor Choices
- Food : Grape Fruit and Drug Interaction
- Food : Vitamins, Minerals and Supplements
- Omega 3 fatty acids
- Vitamin B12 deficiency
- Vitamin D
- Weight Loss
- ORTHOPEDICS
- PAIN
- PATIENTS' RIGHTS
- SKIN
- SLEEP
- SMOKING
- STROKE
- THYROID
- SUBSTANCE ABUSE
- Travel and Vaccination
- TREMOR
- Warfarin Anticoagulation
- OTHER STUFF FOLLOWS
- Fact or Opinion?
- Hippocratic Oath
- FREE ADVICE.......for what its worth!
- LAUGHTER.....is the best medicine
- Physicians Pet Peeves
- PHOTO ALBUM - its not all work!
- Cape Town, South Africa
- Tribute page
- The 100 Club
- Free Wi-Fi
Female : Health Topics
Tackling Menopause’s Side Effects
By Jane E. Brody : NY Times : February 10, 2014
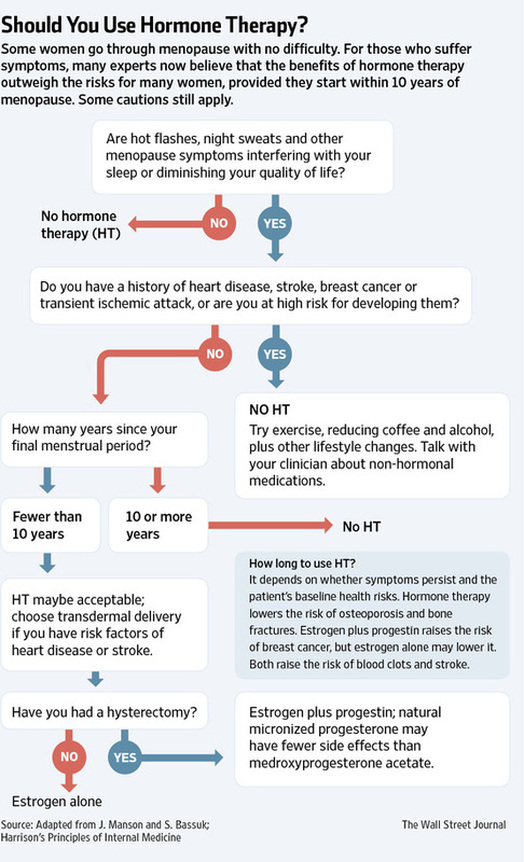
In the-better-late-than-never department, the American College of Obstetricians and Gynecologists has revised its guidelines for effective treatment of the symptoms of menopause.
Published as a practice bulletin for doctors called “Management of Menopausal Symptoms,” the new guidelines recognize that up to three-fourths of women in the United States experience troublesome side effects when their bodies stop producing estrogen as a result of natural or medically induced menopause. The document addresses the most common distressing consequences: hot flushes and vaginal atrophy.
Hot flushes can last for months or even decades, but vaginal problems, if untreated, persist for the remainder of a woman’s life.
Hot flushes can cause drenching, sometimes embarrassing sweating, and seriously disrupt sleep night after night. Vaginal atrophy and the loss of lubrication and elasticity can make sexual encounters painful, depress libido and cause irritation and bleeding during exercise.
You might think the standard treatment would be to administer the hormones that menopausal women are losing. Indeed, supplementation with estrogen was a common practice for decades, and not just for curbing menopausal symptoms. Estrogen was widely promoted as a way to protect women’s health and to keep looking and feeling young well into old age.
But in 2002, a large clinical trial called the Women’s Health Initiative found that the most popular form of hormone replacement, a pill that combined estrogen and synthetic progesterone called Prempro, increased a woman’s risk of heart disease, breast cancer, stroke and blood clots .
The highly publicized results tainted hormone therapy with a broad brush. Although the Women’s Health Initiative was designed primarily to test the popular premise that hormone replacement after menopause protected women’s hearts, the unexpected findings prompted millions of middle-aged and older women to stop using the hormones and kept many millions more from starting them.
Even though the vast majority of the 16,608 participants in the study were older women well past menopause, the findings were widely interpreted to apply to all women going through menopause, even younger women just approaching the end of their fertile years.
Somewhat frenetic experimentation ensued as physicians, drug companies and women themselves searched for effective alternatives to hormone replacement. Various nonhormonal remedies were promoted, from soy foods and black cohosh to exercise and acupuncture. Each had its advocates, but all lacked rigorous scientific evidence for effectiveness.
Those promoting soy-based foods and supplements, for example, cited the low reported rates of menopausal symptoms among Asian women, whose diets are especially rich in soy, which has estrogenic effects. An authoritative review of placebo-controlled studies of plant-based estrogens, however, found no convincing evidence that they were helpful in curbing menopausal hot flushes. (One exception was genistein, a substance in soy, which the researchers said warranted further study.)
The new bulletin, prepared by Dr. Clarisa R. Gracia, an associate professor of obstetrics and gynecology at the University of Pennsylvania, examines the various claims and scores of studies. It offers treatment recommendations based on the best available evidence for preserving the health and well-being of women experiencing menopausal symptoms.
In an interview, Dr. Gracia acknowledged that “there’s a strong placebo effect” when women try one or another suggested remedy for menopausal distress. She admitted that “it’s all for the better” if an innocuous placebo, like a food or supplement, brings relief to some women.
But most women do best when their physicians offer remedies that have been shown to be effective in well-designed studies. And as you might guess, estrogen alone, or in combination with a natural or synthetic progesterone (progestin) for women who still have a uterus, is the “most effective therapy” for curbing hot flushes, the report found.
“Data do not support the use of progestin-only medications, testosterone or compounded bioidentical hormones,” the report also said.
Estrogen with or without progestin can be administered orally or through the skin with a patch, gel or spray. The transdermal route is considered safer: When absorbed through the skin, the hormones bypass the liver, which would otherwise create substances that might raise the risk of heart attack or cancer.
The report emphasizes that treatment with hormones must be individualized and that doctors should prescribe the lowest effective dose for the shortest time needed to relieve hot flushes. But because some women may need hormone therapy to control hot flushes even in their Medicare years, the guidelines recommend “against routine discontinuation of systemic estrogen at age 65.”
Hormone replacement can be risky for some women, especially those who have had breast cancer. Alternatives that have proved helpful include low doses of antidepressants known as selective serotonin reuptake inhibitors (S.S.R.I.’s), like Paxil, and serotonin-norepinephrine reuptake inhibitors (S.N.R.I.’s), like Pristiq. Clonidine, a blood pressure medication, and gabapentin, an anticonvulsant, may also be helpful, though neither is approved by the Food and Drug Administration for menopausal treatment.
The report found little or no data to support the use of herbal remedies, vitamins, phytoestrogens (like isoflavones, soy and red clover) or acupuncture to relieve hot flushes. It did recommend “common sense lifestyle solutions” like dressing in layers, lowering room temperatures, consuming cool drinks, and avoiding alcohol and caffeine. For overweight and obese women, weight loss can also help.
As with hot flushes, vaginal symptoms respond best to estrogen therapy, which can be administered through the mouth or skin or locally via a cream, tablet or ring. Even a low-dose vaginal tablet containing 10 micrograms of estradiol improves symptoms, the report noted.
Low-dose vaginal treatments are administered daily for a week or two at first, then once or twice a week indefinitely as maintenance therapy.
Because small amounts of estrogen used vaginally can enter general circulation, women who have had hormone-sensitive breast cancer are advised to try nonhormonal remedies first.
Many women experience relief of symptoms with lubricants and moisturizers prepared with water or silicone. Lubricants, applied just before intercourse, can reduce friction and pain caused by dryness. Moisturizers are used routinely to relieve dryness, itching, irritation and pain, and to improve elasticity.
Last year, the Food and Drug Administration approved Osphena (ospemifene) to treat vaginal atrophy related to menopause. The drug, taken orally once a day, is a selective estrogen receptor modulator that has estrogenlike effects in the vagina.
But Osphena, too, can promote endometrial growth. It is not recommended for women who have had breast cancer, and it is not intended for long-term use.
No wonder we are all confused!
1991 National Institutes of Health embarks on a study of menopause hormones after observational data suggest that women who use hormones have lower rates of heart disease.
2002 Part of the Women's Health Initiative is stopped after women in the study taking estrogen plus progestin (E+P) show higher rates of heart attack, stroke and breast cancer. Millions of women abandon hormones overnight.
2003 Women taking E+P are not protected from mild memory loss; they are found to be at increased risk for developing dementia.
2004 The second W.H.I. hormone study is stopped one year early because women taking estrogen only show a small increased risk of stroke.
2006 An updated analysis of the estrogen-only trial shows hormone therapy does not increase the risk of breast cancer in postmenopausal women.
2007 Combined data from both hormone trials suggest that timing of therapy may affect risk; hormones may reduce heart disease in women who start therapy closer to menopause.
2009 Women using E+P for more than about five years double their annual risk of breast cancer. That risk is higher than previously thought.
2011 Follow-up of women in the estrogen-only study shows those who took just estrogen had 23 percent fewer breast cancers; younger estrogen users had 46 percent fewer heart attacks.
1991 National Institutes of Health embarks on a study of menopause hormones after observational data suggest that women who use hormones have lower rates of heart disease.
2002 Part of the Women's Health Initiative is stopped after women in the study taking estrogen plus progestin (E+P) show higher rates of heart attack, stroke and breast cancer. Millions of women abandon hormones overnight.
2003 Women taking E+P are not protected from mild memory loss; they are found to be at increased risk for developing dementia.
2004 The second W.H.I. hormone study is stopped one year early because women taking estrogen only show a small increased risk of stroke.
2006 An updated analysis of the estrogen-only trial shows hormone therapy does not increase the risk of breast cancer in postmenopausal women.
2007 Combined data from both hormone trials suggest that timing of therapy may affect risk; hormones may reduce heart disease in women who start therapy closer to menopause.
2009 Women using E+P for more than about five years double their annual risk of breast cancer. That risk is higher than previously thought.
2011 Follow-up of women in the estrogen-only study shows those who took just estrogen had 23 percent fewer breast cancers; younger estrogen users had 46 percent fewer heart attacks.
7 Things You Should Know About Hormone Replacement Therapy
Melinda Beck : WSJ Article : February 3, 2009
A muddle of misinformation keeps clouding the debate over hormone-replacement therapy for women.
Last week, millions of women tuned into "The Oprah Winfrey Show" to hear actress Suzanne Somers sympathize with women suffering from what she called "The Seven Dwarfs of Menopause: Itchy, Bitchy, Sleepy, Sweaty, Bloated, Forgetful and All Dried Up." As she's done in her best-selling books, Ms. Somers, age 62, credited a custom-made blend of "bio-identical" hormones with maintaining her youthful zest and told viewers that the hormone debate boils down to a choice between "restoration versus deterioration."
There was little discussion of potential risks of HRT. The compounding pharmacies that make up such custom blends of hormones without oversight by the Food and Drug Administration often claim their products are so natural that they confer the benefits of hormone replacement (from restoring sleep, mood, memory and sexiness to protecting against osteoporosis) without the risks.
Millions of women abandoned menopause hormones after the big Women's Health Initiative trial was halted early in 2002 amid signs that they increased the risk of heart attack and stroke. A growing number of experts now believe that the women in the WHI -- average age 63 -- do not reflect the typical women entering menopause, and that the same risks may not apply to younger women.
Even so, women seeking safer alternatives have turned to "bio-identicals" -- a trend that worries mainstream medical groups. "Women who were afraid after the WHI, as were their doctors, are going to alternative approaches that have little or no scientific information behind them," says Margaret Weirman, a professor of medicine at the University of Colorado Denver and a spokeswoman for the Endocrine Society, a professional organization devoted to hormone research. "These women may be putting themselves at much higher levels of risk."
Amid all the confusion, here are seven things women should know about the HRT debate now:
1) 'Bio-identical' hormones are available in FDA-approved forms.
Though many experts dismiss "bio-identical" as a meaningless term, proponents use it to mean hormones with the same molecular structure as those that women's bodies make. The main one lost at menopause is estradiol, which affects functions throughout the female body, from skin to bones, hearts and brains. Chemically equivalent estradiol is available in many FDA-approved pills, patches, creams and gels from traditional drug companies, generally made from the exact same plant sources that compounding pharmacies use. What's more, the FDA-approved varieties are covered by insurance, unlike compounded blends that can cost hundreds of dollars a month.
A growing number of doctors prescribe these estradiol-based products instead of Premarin, the estrogen made from horse urine that was used in the WHI. Many also prefer natural progesterone, available in FDA-approved Prometrium, to the synthetic form that was used in the WHI. But there is little evidence comparing one HRT variety against another.
2) Hormones from compounding pharmacies aren't safer than conventional HRT.
Compounded drugs don't carry warnings or list side effects on their labels, but that's because they are not made under FDA scrutiny. In fact, they can vary greatly in strength and potency and little is known about how they release active ingredients over time. "We don't know if it comes out in peaks and valleys or continuously," says Dr. Weirman. "Some people may be getting very high doses, and some people may be getting very little or none."
The International Academy of Compounding Pharmacists contends that its members perform a valuable service in making drugs and strengths that aren't commercially available, that they are providing women with freedom of choice in health-care decisions and that much of the criticism is coming from groups funded by makers of traditional HRT.
3) Don't trust saliva tests.
Some compounding pharmacies use saliva tests to monitor women's hormone levels and develop custom blends. But many experts say such tests (which can cost hundreds of dollars) are unreliable and lack uniform standards.
Blood tests are more accurate -- but monitoring how women feel is just as key. Many doctors believe that HRT should be used mainly to treat actual symptoms such as hot flashes, mood swings, sleep problems, foggy thinking and other symptoms, rather than arbitrary blood levels since individual ranges vary widely. FDA-approved estradiol products are available in a wide variety of strengths that can be tapered as a woman's symptoms change.
Hormone Options
FDA-approved products with estradiol:
There's a growing consensus that the risks and benefits are different for younger and older women, and that for women who start HRT shortly after menopause, the benefits may outweigh the risks. Women in the WHI who were 20 years past menopause had a 71% higher risk of heart attack on estrogen and progesterone than those taking placebos, but women closer to menopause had an 11% lower risk of heart problems. One theory is that estrogen helps keep healthy blood vessels supple, but make atherosclerosis worse once it has set in.
Similarly, HRT seems to help preserve thinking ability when started just after menopause, but it may hasten the progression of pre-existing memory problems when started later in life, writes JoAnn E. Manson, a Harvard Medical School professor who was a lead investigator on both the WHI and the long-running Nurses' Health Study, in her new book, "Hot Flashes, Hormones and Your Health."
HRT was associated with a lower risk of fractures and colorectal cancer regardless of age. The WHI did not assess whether HRT improved quality-of-life issues such as mood, sleep and hot flashes. Women experiencing such symptoms were excluded from the study on the grounds that immediate effects might prompt some to guess whether they were in the control or placebo group.
5) The increased risk of breast cancer appears related to progesterone rather than estrogen.
Women taking both estrogen and progesterone in the WHI had eight more cases of breast cancer per 10,000 than the control group; women taking estrogen alone had six fewer cases. Women who still have a uterus need some progesterone to guard against uterine cancer, but many doctors now try to give the lowest dose possible to prevent a build-up of uterine lining.
6) Estrogen applied to the skin, in patch, cream or gel form, may have a lower risk of blood clots and strokes than in pill form.
A large study in France published in the Lancet found that women taking estrogen in pill form were three times as likely to develop blood clots than non-users, while women using the estradiol patch had no increased risk. But more study is needed to determine this conclusively.
7) Stay tuned.
Several of these new theories are being tested in another trial called KEEPS (for Kronos Early Estrogen Prevention Study) that is comparing 720 newly menopausal women on oral Premarin, an estrogen patch or placebo. Investigators will monitor their arteries, as well as quality-of-life aspects like mood, fatigue, sleep, bone health and cognition.
In the meantime, women entering menopause should discuss all the risks and benefits with their doctors, as well as their symptoms, health and family history, and make an individual, informed decision.
For Some in Menopause, Hormones May Be Only Option
By Tara Parker-Pope : NY Times : August 15, 2011
For women hoping to combat the symptoms of menopause with nonprescription alternatives like soy and flaxseed supplements, recent studies have held one disappointment after another.
Last week, a clinical trial found that soy worked no better than a placebo for hot flashes and had no effect on bone density. That followed a similar finding about hot flashes from a clinical trial of flaxseed.
“We wish we could have told women that, yes, they work,” said Dr. Silvina Levis, director of the osteoporosis center at the University of Miami, who led the soy study. “Now we have shown that they don’t.”
Before 2002 women were routinely treated with the prescription hormones estrogen and progestin, which rapidly fell out of favor after the landmark Women’s Health Initiative study showed that older women who used them had a heightened risk of heart attacks and breast cancer.
But now some doctors are arguing that those risks do not apply to the typical woman with menopause symptoms, and even some longtime critics of hormone treatment are suggesting that it be given another look for women suffering from severe symptoms.
Study after study has shown that many nondrug treatments — black cohosh, red clover, botanicals, and now soy and flaxseed — simply don’t work. Prescription medicines, including antidepressants, the blood pressure drug clonidine and the seizure drug gabapentin may have some benefit, but many women cannot tolerate the side effects.
“There is no alternative treatment that works very well, whether it’s a drug or over-the-counter herbal preparation,” said Dr. Deborah Grady, associate dean for clinical and translational research at the University of California, San Francisco.
About 75 percent of menopausal women experience hot flashes. Depending on the woman, symptoms can be mild, occurring only a few times a week, or moderate, occurring several times a day. Many women with mild to moderate symptoms cope without needing further treatment. But about a third of women have severe symptoms, experiencing 10 to 20 hot flashes day and night that disrupt their workdays and interfere with sleep.
While doctors often reassure women that it will all be over in just a few years, a May report in the journal Obstetrics and Gynecology found that during one long-term study of women, menopausal hot flashes recurred for some women, for 10 years or more.
A hot flash is usually described as a sudden warmth first felt in the face and neck. Hot flashes can turn a woman’s face red and lead to excessive sweating and then chills. On the Web site MinniePauz.com, women describe feeling lightheaded and dazed, with heart palpitations and anxiety.
“Whoosh, a rush of heat originating from the core of your body,” is how one woman put it. “Hair goes lank and you know that even if you stripped naked and ran down the high street waving your arms to fan your body, well you still wouldn’t get cool because the heat is inside you, not outside.”
The exact cause of hot flashes is not known, but it is believed that menopause disrupts the function of the hypothalamus, which is essentially the body’s thermostat. As a result, even small changes in body temperature that would normally go unnoticed can set off hot flashes.
Among prescription drug treatments, the most effective may be antidepressants, including Effexor, Paxil and Pristiq, which have been shown to reduce hot flashes by as much as 60 percent, doctors say. Antidepressants are particularly useful for women with breast cancer or blood-clot disorders who do not have the option of taking a hormone drug.
But some doctors say they are frustrated by the message given to many women that they must seek an alternative treatment to prescription hormones. Dr. Holly Thacker, director of the center of specialized women’s health at the Cleveland Clinic, said that for many women the benefits of effective hormone treatment would outweigh the risks and that they should not be scared off from considering the drugs.
“It would be like telling someone with insulin-dependent diabetes that they should try to use other things besides insulin,” she said. “I see women look to alternative agents and coming in with bags of things, and they have no idea what they are putting into their body. There has been so much misinformation, and they are confused.”
Dr. Grady, a longtime critic of widespread hormone use, said doctors and women appeared to be less tolerant of risks associated with hormones than of those with other drugs, even though menopause symptoms can be just as intolerable as migraine pain or other health problems.
“Somehow we’re quite willing to take a migraine drug with its associated adverse effects because it works so well, but we’re not willing to take estrogen,” she said. “We worry about the adverse effects associated with estrogen, but the important adverse effects are reasonably uncommon.
“The question is whether a woman is willing to trade off that risk for a very effective treatment for symptoms that are otherwise ruining her life.”
Subtle Science: Heading Off Heart Attacks in Women
By Melinda Beck
WSJ Article : November 25, 2008
Men's and women's hearts are different -- in ways that doctors are still working to understand.
A recent government report adds more mystery: According to the federal Agency for Healthcare Research and Quality, or AHRQ, women are far more likely than men to be hospitalized for chest pain for which doctors can't find a cause.
In 2006, the latest data available, 477,000 women were discharged from U.S. hospitals with a diagnosis of nonspecific chest pain, compared with 379,000 men. That diagnosis is often given to patients who are admitted for a possible heart attack that turns out not to be one.
Heart disease is the No. 1 killer of both sexes, claiming more lives each year than all cancers combined. More women than men have died from heart disease in the U.S. every year since 1984, and they are twice as likely as men to die after a heart attack, according to the National Coalition for Women with Heart Disease.
But heart attacks often look different in women than men. While both frequently report chest pain, pressure or tightness, women more often have subtle signs instead, including dizziness, nausea, breathlessness, aches in the back, shoulders or abdomen, sudden weakness or fatigue or an overwhelming feeling of doom. A 2003 study found that 95% of women who had heart attacks started feeling some of those symptoms a month or more before.
"Women are very into their bodies -- they know when they aren't feeling well," says Nieca Goldberg, medical director of New York University's Women's Heart Program. "That's the time to call your doctor, when there's still time to prevent a heart attack."
Could some of those nonspecific chest pains in the AHRQ report have been early warning signs? There's no way of knowing, since the statistics don't track whether patients returned with a heart attack later.
Warning for Women
Chest pain is a common symptom in heart attacks, but women may instead have:
But even when women do have such diagnostic tests, doctors are less likely to find CAD. "Women with chest pain are much more likely to have normal coronary arteries," says Rita F. Redberg, director of Women's Cardiovascular Services at the University of California -- San Francisco School of Medicine. "The truth is, we don't always find out what causes it."
One theory is that some chest pain in women may be due to microvascular disease -- blockages in tiny vessels that can't be seen on an angiogram. Such blockages are also too small to be opened with stents or angioplasty, but can be treated with beta blockers and nitroglycerine.
Given the growing awareness that heart attacks present differently in women, the AHRQ data may indicate that doctors are treating women with chest pain more proactively than they used to.
"Women's chest pain is increasingly taken more seriously -- even if it's atypical -- so doctors may have a lower threshold for admitting them," says cardiologist Edmund M. Herrold, a professor of medicine at the State University of New York-Downstate Medical Center in Brooklyn. "I think that's a good thing. In the past, women who should have been admitted were being sent home."
Should women think twice about getting help if they're having chest pain? "We're always trying to draw that line fine -- not wanting people to ignore symptoms, but not thinking it's a heart attack everytime they get a twinge," says Dr. Redberg.
Studies show that women wait longer than men before going to the hospital -- in part because the symptoms may be subtle, and in part because they don't want to "bother" doctors if nothing's wrong. But two-thirds of women who have heart attacks never make it to the hospital.
If you have any of the symptoms for more than a few minutes, seek help quickly. "Don't sit home and wonder," says Dr. Goldberg, who sometimes has to beg patients who call her in the middle of the night to go to the hospital.
She also suggests calling 911 rather than going on your own: "You'll get taken care of faster in the emergency room if you arrive by ambulance. The hospital can call the doctor for you."
Beyond Hysterectomy: Alternative Options for Heavy Bleeding
By Carolyn Sayre
NY Times Article : October 3, 2008
In Brief:
Heavy or prolonged bleeding affects nearly one in five women ages 35 to 49.
Hysterectomy was the treatment of choice 20 years ago, but new options like the Mirena intrauterine device and medications often make surgery unnecessary.
Today, many women with menorrhagia continue to suffer because they are unaware of new treatment options.
It’s rare to find a woman who has never had any trouble with her period. For most, though, the hassles are fairly minor — irritability, mild cramping and the occasional surprise visit. But for the nearly one in five women ages 35 to 49 who suffer from menorrhagia — heavy or prolonged bleeding — their menstrual cycle puts life on hold every month.
“It controlled my life,” said Stefanie Haase, 37, an X-ray technician from Sodus, N.Y., who started bleeding uncontrollably two years ago because of a large fibroid. “I had to carry an extra set of clothes with me at all times. I couldn’t drive without messing up the car, or plan a vacation. My life revolved around my period.”
Twenty years ago, women like Ms. Haase did not have many options. For the nearly 40 percent of women whose abnormal bleeding stemmed from anatomical problems like fibroids and polyps, an invasive surgical procedure to remove the noncancerous growths, called a myomectomy, was often helpful but did not always stop the bleeding. For the majority of patients, the outlook was pretty unpleasant. High-dose hormones caused a host of side effects like rapid weight gain. A procedure called dilation and curettage, or D&C, which scraped away the lining of the uterus, typically failed to stop the bleeding for more than a few months.
Ms. Haase would have quickly found herself out of options and, like many women living with menorrhagia back then, her last resort would likely have been a hysterectomy — major surgery to remove the uterus and sometimes other reproductive organs as well. But thanks to new treatments and techniques, all that has changed.
“Women no longer need to choose between suffering and major surgery,” said Dr. Glenn Schattman, a reproductive surgeon and associate professor of obstetrics and gynecology at the New York Presbyterian Hospital/Weill Medical College of Cornell. “Today, the key is avoiding major surgery, and thankfully, the overwhelming majority of patients can now be treated with minimally invasive procedures or medication.”
The changes began in 1987, when the Food and Drug Administration approved a laser that could destroy the lining of the uterus by heating it. The laser outpatient procedure that became known as endometrial ablation was a retreat from the finality of a hysterectomy, which not only removes major organs but, back then, also required up to two months of recovery time.
A few years later, physicians began to refine the procedure, developing more sophisticated techniques like endometrial resection, which removes the uterine lining entirely.
“Women who lived 20 years ago were pushed onto a track with very few off-ramps,” said Dr. Morris Wortman, director of the Center for Menstrual Disorders & Reproductive Choice in Rochester. “Endometrial ablation changed that by obviating the need for hysterectomy. It gave women choices.”
Dr. Wortman notes that endometrial ablation stops bleeding in nearly half of women and significantly reduces bleeding in another 40 percent.
But like most procedures, endometrial ablation and resection also have downsides. About 15 percent of patients still need a hysterectomy, and while the uterus remains intact after the endometrial procedures, a woman’s chance of conception is destroyed.
Medical therapies now help eliminate some of the fertility concerns. In the early ’90s, low-dose birth control pills began to be prescribed for women with heavy bleeding who did not have structural problems. Perhaps the biggest breakthrough came in 2001, when the F.D.A. approved Mirena, an intrauterine birth control device that lightens bleeding in more than 80 percent of women.
Today, most physicians consider the fertility-preserving Mirena IUD to be the first-line treatment for heavy bleeding in women with normal anatomy.
“It works beautifully,” said Dr. Linda Bradley, director of hysteroscopic services for the department of obstetrics and gynecology at the Cleveland Clinic’s Center for Menstrual Disorders and Fibroids. “Women who take it either don’t get their period or bleed very lightly.”
That was certainly the case for Sheila Woodruff, 45, of Cleveland, who started using the Mirena IUD last spring. After two years of heavy bleeding that lasted up to 10 days every month and caused her to change her clothes every couple hours, she finally had enough.
The Mirena IUD, she said, “was unbelievable.”
“My period magically became normal again.”
In a small percentage of women, the device causes side effects like spotting or abdominal pain and may need to be removed.
And for women with anatomical problems like fibroids and polyps, minimally invasive procedures like laparoscopic myomectomy have reduced recovery time significantly. And uterine fibroid embolization, a nonsurgical technique that shrinks or kills the growths by cutting off their blood supply, has helped certain patients with structural problems avoid surgery altogether.
Now that alternative treatments to hysterectomy have been developed, physicians say their biggest challenge is raising awareness and acceptance among women, many of whom still fear that major surgery is their only option.
“I can’t believe how many women are essentially bedridden during their periods for a year or two before they see a doctor,” Dr. Schattman said. “They are still scared that heavy bleeding means they will need a hysterectomy.”
According to the Centers for Disease Control and Prevention, 20 million women now living in the United States have had a hysterectomy. “That number should be lower,” Dr. Wortman said. “And it would be if more women understood all of their options.”
Despite the fact that many physicians say the Mirena device is safe and remarkably effective, women who remember the infamous side effects of the Dalkon Shield, a popular IUD used in the ’70s, still shy away from using it. What’s more, techniques like endometrial ablation and resection have only really begun to catch on in the last 10 years or so as technological advancements have made it easier for less-specialized gynecologists to perform the procedures.
Ms. Haase, for one, waited it out for nearly two years before she underwent surgery. Unlike most patients with fibroids, she underwent both a myomectomy and endometrial resection to stop her bleeding.
“My mother had a hysterectomy when she was 33, so I figured that was my fate,” she said. “But there are so many options out there. I only wish I had known that two years ago.”
More doctors are challenging the convention of removing the cervix during a hysterectomy
By Laura Johannes
WSJ Article : February 24, 2009
For decades, surgeons performing a hysterectomy, or removal of the uterus, typically cut out the woman's cervix as well. If it were left in, doctors reasoned, it could develop cancer.
Now, a growing number of gynecologists are marketing "cervix-sparing" hysterectomies if cancer isn't present. The chance of cervical cancer is fairly low, and Pap-smear screening will catch most cases, these doctors say. And leaving the cervix untouched reduces the risk of surgical damage to the bladder and nearby nerves, and may even allow a woman to enjoy a better sex life long term, say doctors who perform these procedures.
Not so fast, argues the American College of Obstetricians and Gynecologists. The influential nonprofit doctors' group argued in a 2007 report that evidence was lacking that sparing the cervix improves one's sex life or bladder function. And if a growth develops, removing the cervix alone carries higher risk, including infection, than a hysterectomy, the group says. "It's not appropriate for surgeons to recommend it as a superior technique," says Denise Jamieson, chairman of the committee that wrote the report.
Cutting Less
More doctors are challenging the convention of removing the cervix during a hysterectomy. Here's why:
Now the tide appears to be turning. In 2006, 9.7% of U.S. inpatient hysterectomies, which account for more than 90% of the procedures, preserved the cervix, compared with just 1.7% a decade earlier, according to federal data.
The cervix is a small, doughnut-shaped mass of tissue at the base of the uterus. Downsides to keeping it include the need to continue regular Pap smears and the possibility of continued menstrual bleeding or spotting, if the surgeon doesn't succeed in removing all of the uterus.
"When you give women the choice, and you tell them the pros and cons, many of them find the idea of keeping the cervix very appealing," says Seth Kivnick, a physician at Kaiser Foundation Hospital in West Los Angeles. Three-quarters of women without cancer choose to keep the cervix when having a hysterectomy at the hospital, he says.
Some researchers believe that for at least some women, the cervix may contribute to sexual pleasure; doctors also say leaving it in place makes it easier to avoid unwittingly shortening the vaginal canal. A 212-patient Finnish study from 1983 found pain upon intercourse pre-hysterectomy was better relieved by a cervix-sparing procedure. A parallel study, involving the same women, found the frequency of orgasms decreased in women who had their cervix removed but not in those who didn't.
Dr. Jamieson, of the American College of Obstetricians and Gynecologists, says the orgasm finding didn't reach statistical significance. Also, the study designs were flawed, in part because the women weren't assigned randomly to the different procedures, she says.
Other studies have failed to validate a positive role for sparing the cervix. A 2005 study of 135 women found no difference in sexual functioning or quality of life between women who had the cervix-sparing and regular hysterectomies. "It's not to say that an individual woman may not be in a better place, but on average it's not true," says lead author Miriam Kuppermann, an epidemiologist at the University of California, San Francisco.
One limitation of the research is that various studies, including Dr. Kuppermann's, compared hysterectomies done with a large abdominal incision. Many surgeons pushing the cervix-sparing procedure do a minimally invasive version involving only three or four tiny incisions, which helps shorten recovery times. Removal of both the uterus and the cervix can also be performed minimally invasively, but this option isn't yet widely available. In another type of procedure, called "vaginal hysterectomy," no abdominal incisions are made and the uterus is removed through the vagina; the cervix generally must be removed. Women whose Pap smears recently found lesions that could be pre-cancerous are generally advised to have the cervix removed during a hysterectomy. Some physicians won't leave the cervix in place if a woman tests positive for strains of human papillomavirus that can cause cervical cancer.
A Dip in the Sex Drive, Tied to Menopause
By Jane E. Brody
NY Times Article ; March 31, 2009
Concern about the safety of hormone replacement has all but obscured one of the most pressing concerns for women of a certain age: the effects of menopause on their sex lives. Many are reluctant to ask their doctors a question uppermost in their minds: “What has happened to my desire for sex and my ability to enjoy it?”
With fully a third of their lives ahead of them, but with little or none of the hormones that fostered what may have been a robust sex life, many postmenopausal women experience diminished or absent sexual desire, difficulty becoming aroused or achieving orgasm, or pain during intercourse caused by menopause-related vaginal changes.
Sometimes the reasons for these problems go beyond hormones. Some women may consider themselves less sexually attractive as their bodies change with age, or they have partners who have lost interest in sex or the ability to perform reliably.
But for most postmenopausal women, hormone-related changes are the primary factors that interfere with sexual satisfaction. My friend Linda, for example, who lives in Pittsburgh, was 52 years old and recently married when her vibrant interest in sex suddenly plummeted, leading to a frantic search for a way to restore it.
A more common situation is described by Pat Wingart and Barbara Kantrowitz in their informative book, “Is It Hot in Here or Is It Me?” (Workman, 2006): “You’re not in the mood a lot of the time. Most nights, you just wish your partner would roll over and go to sleep. When you do feel like a little action, it takes forever to get warmed up. Sometimes sex is more painful than pleasurable.”
Common Changes
Unlike Linda, who had an abrupt change in desire, many women report a gradual decline in sexual desire as they age. In a survey of 580 menopausal women conducted by Siecus, the Sexuality Information and Education Council of the United States, 45 percent reported a decrease in sexual desire after menopause, 37 percent reported no change and 10 percent reported an increase.
Although individual experiences certainly vary, “Changes in arousal clearly are associated with menopause,” according to a 2007 article in The Journal of the American Medical Association. The author, Dr. Jennifer E. Potter of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, said physical factors include less blood flow to genital organs, a decrease in vaginal lubrication and a decreased response to touch.
Women can achieve orgasm throughout their lives, but they typically need more direct, more intense and longer stimulation of the clitoris to reach a climax, Dr. Potter noted.
Another common experience is a diminished intensity of orgasm and painful uterine contractions after orgasm, although the women surveyed by Siecus said over all that they remained satisfied with sex.
Yet as Dr. Potter put it, “What might be a satisfying sexual life for one woman may seem woefully inadequate to another,” adding that what a woman expects from her sex life can make a difference. She cited the findings of various large surveys: “Only one-third to one-half of women who report decreased desire or response believe they have a problem or feel distress for which they would like help.”
So what happens to a woman’s body when levels of sex hormones fall?
Although estrogen is a woman’s predominant hormone before menopause, testosterone, produced in women primarily by the ovaries and adrenal glands, is considered the libido hormone for both men and women.
Testosterone levels in women decline by about 50 percent between the ages of 20 and 45, and the amount of testosterone produced continues to decline gradually as women age. While menopause itself has no direct effect on testosterone production, surgical removal of the ovaries can cause an abrupt drop in this hormone and accompanying sexual desire, especially for women who have not gone through natural menopause.
For some women, the increased ratio of testosterone to estrogen that occurs after menopause gives their sex drive a boost, Ms. Wingart and Ms. Kantrowitz point out.
But for most women, the menopausal effects of low levels of estrogen are the primary deterrents to sexual pleasure. In addition to the infamous hot flashes, changes in the vagina and vulva can have serious effects on the sexual experience.
¶With little or no estrogen, vaginal walls become dry, thin and less elastic, causing pain during penetration.
¶Diminished blood flow to the genital area means it can take much longer for a woman to feel aroused.
¶The anticipation of painful uterine contractions with orgasm can be a turnoff.
¶A leakage of urine some women experience during sex can prompt them to avoid it.
Helpful Treatments
Linda, who asked that her last name not be used, said she was more concerned about reviving her sex life than a possible increased risk of hormone-induced cancer or heart disease. A prescription of the drug Estratest, which combines estrogen and testosterone, solved her problem.
But taking estrogen orally is not recommended for women who have had breast cancer or are at high risk for developing it. Also, to protect the uterus against cancer, estrogen should be combined with a progestin.
An alternative that works for some is vaginal application of a little estrogen via a cream, ring or tablet, which keeps the hormone from passing through the liver and diminishes the amount that enters the bloodstream.
Gynecologists concerned about safety are more likely to recommend a non-oil-based lubricant. Besides such popular products as K-Y jelly, Ms. Wingart and Ms. Kantrowitz suggest several longer-lasting products that have an adhesive quality, including Replens, K-Y Long-Lasting Vaginal Moisturizer and Astroglide Silken Secret. The authors said “women who have intercourse regularly seem to generate more lubrication than those who do it less frequently.”
Infrequent intercourse or prolonged periods without it can result in a narrowing of the vagina that can be countered by the use of lubricated vaginal dilators. For women whose sex lives are disrupted by lack of a partner, the authors recommend self-stimulation. Dr. Potter suggested that even for women with partners, a vibrator or small battery-powered vacuum pump can aid in arousal.
While a Viagra-like drug is not yet an option for women, use of the antidepressant bupropion (Wellbutrin at 300 milligrams a day) may improve sexual arousal and satisfaction in women who are not depressed. And Dr. Potter pointed out that remaining physically fit can also help.
Melinda Beck : WSJ Article : February 3, 2009
A muddle of misinformation keeps clouding the debate over hormone-replacement therapy for women.
Last week, millions of women tuned into "The Oprah Winfrey Show" to hear actress Suzanne Somers sympathize with women suffering from what she called "The Seven Dwarfs of Menopause: Itchy, Bitchy, Sleepy, Sweaty, Bloated, Forgetful and All Dried Up." As she's done in her best-selling books, Ms. Somers, age 62, credited a custom-made blend of "bio-identical" hormones with maintaining her youthful zest and told viewers that the hormone debate boils down to a choice between "restoration versus deterioration."
There was little discussion of potential risks of HRT. The compounding pharmacies that make up such custom blends of hormones without oversight by the Food and Drug Administration often claim their products are so natural that they confer the benefits of hormone replacement (from restoring sleep, mood, memory and sexiness to protecting against osteoporosis) without the risks.
Millions of women abandoned menopause hormones after the big Women's Health Initiative trial was halted early in 2002 amid signs that they increased the risk of heart attack and stroke. A growing number of experts now believe that the women in the WHI -- average age 63 -- do not reflect the typical women entering menopause, and that the same risks may not apply to younger women.
Even so, women seeking safer alternatives have turned to "bio-identicals" -- a trend that worries mainstream medical groups. "Women who were afraid after the WHI, as were their doctors, are going to alternative approaches that have little or no scientific information behind them," says Margaret Weirman, a professor of medicine at the University of Colorado Denver and a spokeswoman for the Endocrine Society, a professional organization devoted to hormone research. "These women may be putting themselves at much higher levels of risk."
Amid all the confusion, here are seven things women should know about the HRT debate now:
1) 'Bio-identical' hormones are available in FDA-approved forms.
Though many experts dismiss "bio-identical" as a meaningless term, proponents use it to mean hormones with the same molecular structure as those that women's bodies make. The main one lost at menopause is estradiol, which affects functions throughout the female body, from skin to bones, hearts and brains. Chemically equivalent estradiol is available in many FDA-approved pills, patches, creams and gels from traditional drug companies, generally made from the exact same plant sources that compounding pharmacies use. What's more, the FDA-approved varieties are covered by insurance, unlike compounded blends that can cost hundreds of dollars a month.
A growing number of doctors prescribe these estradiol-based products instead of Premarin, the estrogen made from horse urine that was used in the WHI. Many also prefer natural progesterone, available in FDA-approved Prometrium, to the synthetic form that was used in the WHI. But there is little evidence comparing one HRT variety against another.
2) Hormones from compounding pharmacies aren't safer than conventional HRT.
Compounded drugs don't carry warnings or list side effects on their labels, but that's because they are not made under FDA scrutiny. In fact, they can vary greatly in strength and potency and little is known about how they release active ingredients over time. "We don't know if it comes out in peaks and valleys or continuously," says Dr. Weirman. "Some people may be getting very high doses, and some people may be getting very little or none."
The International Academy of Compounding Pharmacists contends that its members perform a valuable service in making drugs and strengths that aren't commercially available, that they are providing women with freedom of choice in health-care decisions and that much of the criticism is coming from groups funded by makers of traditional HRT.
3) Don't trust saliva tests.
Some compounding pharmacies use saliva tests to monitor women's hormone levels and develop custom blends. But many experts say such tests (which can cost hundreds of dollars) are unreliable and lack uniform standards.
Blood tests are more accurate -- but monitoring how women feel is just as key. Many doctors believe that HRT should be used mainly to treat actual symptoms such as hot flashes, mood swings, sleep problems, foggy thinking and other symptoms, rather than arbitrary blood levels since individual ranges vary widely. FDA-approved estradiol products are available in a wide variety of strengths that can be tapered as a woman's symptoms change.
Hormone Options
FDA-approved products with estradiol:
- Alora
- Climara
- Vivelle
- Estraderm
- Estrace
- Estrogel
- Prometrium
- Crinone
There's a growing consensus that the risks and benefits are different for younger and older women, and that for women who start HRT shortly after menopause, the benefits may outweigh the risks. Women in the WHI who were 20 years past menopause had a 71% higher risk of heart attack on estrogen and progesterone than those taking placebos, but women closer to menopause had an 11% lower risk of heart problems. One theory is that estrogen helps keep healthy blood vessels supple, but make atherosclerosis worse once it has set in.
Similarly, HRT seems to help preserve thinking ability when started just after menopause, but it may hasten the progression of pre-existing memory problems when started later in life, writes JoAnn E. Manson, a Harvard Medical School professor who was a lead investigator on both the WHI and the long-running Nurses' Health Study, in her new book, "Hot Flashes, Hormones and Your Health."
HRT was associated with a lower risk of fractures and colorectal cancer regardless of age. The WHI did not assess whether HRT improved quality-of-life issues such as mood, sleep and hot flashes. Women experiencing such symptoms were excluded from the study on the grounds that immediate effects might prompt some to guess whether they were in the control or placebo group.
5) The increased risk of breast cancer appears related to progesterone rather than estrogen.
Women taking both estrogen and progesterone in the WHI had eight more cases of breast cancer per 10,000 than the control group; women taking estrogen alone had six fewer cases. Women who still have a uterus need some progesterone to guard against uterine cancer, but many doctors now try to give the lowest dose possible to prevent a build-up of uterine lining.
6) Estrogen applied to the skin, in patch, cream or gel form, may have a lower risk of blood clots and strokes than in pill form.
A large study in France published in the Lancet found that women taking estrogen in pill form were three times as likely to develop blood clots than non-users, while women using the estradiol patch had no increased risk. But more study is needed to determine this conclusively.
7) Stay tuned.
Several of these new theories are being tested in another trial called KEEPS (for Kronos Early Estrogen Prevention Study) that is comparing 720 newly menopausal women on oral Premarin, an estrogen patch or placebo. Investigators will monitor their arteries, as well as quality-of-life aspects like mood, fatigue, sleep, bone health and cognition.
In the meantime, women entering menopause should discuss all the risks and benefits with their doctors, as well as their symptoms, health and family history, and make an individual, informed decision.
For Some in Menopause, Hormones May Be Only Option
By Tara Parker-Pope : NY Times : August 15, 2011
For women hoping to combat the symptoms of menopause with nonprescription alternatives like soy and flaxseed supplements, recent studies have held one disappointment after another.
Last week, a clinical trial found that soy worked no better than a placebo for hot flashes and had no effect on bone density. That followed a similar finding about hot flashes from a clinical trial of flaxseed.
“We wish we could have told women that, yes, they work,” said Dr. Silvina Levis, director of the osteoporosis center at the University of Miami, who led the soy study. “Now we have shown that they don’t.”
Before 2002 women were routinely treated with the prescription hormones estrogen and progestin, which rapidly fell out of favor after the landmark Women’s Health Initiative study showed that older women who used them had a heightened risk of heart attacks and breast cancer.
But now some doctors are arguing that those risks do not apply to the typical woman with menopause symptoms, and even some longtime critics of hormone treatment are suggesting that it be given another look for women suffering from severe symptoms.
Study after study has shown that many nondrug treatments — black cohosh, red clover, botanicals, and now soy and flaxseed — simply don’t work. Prescription medicines, including antidepressants, the blood pressure drug clonidine and the seizure drug gabapentin may have some benefit, but many women cannot tolerate the side effects.
“There is no alternative treatment that works very well, whether it’s a drug or over-the-counter herbal preparation,” said Dr. Deborah Grady, associate dean for clinical and translational research at the University of California, San Francisco.
About 75 percent of menopausal women experience hot flashes. Depending on the woman, symptoms can be mild, occurring only a few times a week, or moderate, occurring several times a day. Many women with mild to moderate symptoms cope without needing further treatment. But about a third of women have severe symptoms, experiencing 10 to 20 hot flashes day and night that disrupt their workdays and interfere with sleep.
While doctors often reassure women that it will all be over in just a few years, a May report in the journal Obstetrics and Gynecology found that during one long-term study of women, menopausal hot flashes recurred for some women, for 10 years or more.
A hot flash is usually described as a sudden warmth first felt in the face and neck. Hot flashes can turn a woman’s face red and lead to excessive sweating and then chills. On the Web site MinniePauz.com, women describe feeling lightheaded and dazed, with heart palpitations and anxiety.
“Whoosh, a rush of heat originating from the core of your body,” is how one woman put it. “Hair goes lank and you know that even if you stripped naked and ran down the high street waving your arms to fan your body, well you still wouldn’t get cool because the heat is inside you, not outside.”
The exact cause of hot flashes is not known, but it is believed that menopause disrupts the function of the hypothalamus, which is essentially the body’s thermostat. As a result, even small changes in body temperature that would normally go unnoticed can set off hot flashes.
Among prescription drug treatments, the most effective may be antidepressants, including Effexor, Paxil and Pristiq, which have been shown to reduce hot flashes by as much as 60 percent, doctors say. Antidepressants are particularly useful for women with breast cancer or blood-clot disorders who do not have the option of taking a hormone drug.
But some doctors say they are frustrated by the message given to many women that they must seek an alternative treatment to prescription hormones. Dr. Holly Thacker, director of the center of specialized women’s health at the Cleveland Clinic, said that for many women the benefits of effective hormone treatment would outweigh the risks and that they should not be scared off from considering the drugs.
“It would be like telling someone with insulin-dependent diabetes that they should try to use other things besides insulin,” she said. “I see women look to alternative agents and coming in with bags of things, and they have no idea what they are putting into their body. There has been so much misinformation, and they are confused.”
Dr. Grady, a longtime critic of widespread hormone use, said doctors and women appeared to be less tolerant of risks associated with hormones than of those with other drugs, even though menopause symptoms can be just as intolerable as migraine pain or other health problems.
“Somehow we’re quite willing to take a migraine drug with its associated adverse effects because it works so well, but we’re not willing to take estrogen,” she said. “We worry about the adverse effects associated with estrogen, but the important adverse effects are reasonably uncommon.
“The question is whether a woman is willing to trade off that risk for a very effective treatment for symptoms that are otherwise ruining her life.”
Subtle Science: Heading Off Heart Attacks in Women
By Melinda Beck
WSJ Article : November 25, 2008
Men's and women's hearts are different -- in ways that doctors are still working to understand.
A recent government report adds more mystery: According to the federal Agency for Healthcare Research and Quality, or AHRQ, women are far more likely than men to be hospitalized for chest pain for which doctors can't find a cause.
In 2006, the latest data available, 477,000 women were discharged from U.S. hospitals with a diagnosis of nonspecific chest pain, compared with 379,000 men. That diagnosis is often given to patients who are admitted for a possible heart attack that turns out not to be one.
Heart disease is the No. 1 killer of both sexes, claiming more lives each year than all cancers combined. More women than men have died from heart disease in the U.S. every year since 1984, and they are twice as likely as men to die after a heart attack, according to the National Coalition for Women with Heart Disease.
But heart attacks often look different in women than men. While both frequently report chest pain, pressure or tightness, women more often have subtle signs instead, including dizziness, nausea, breathlessness, aches in the back, shoulders or abdomen, sudden weakness or fatigue or an overwhelming feeling of doom. A 2003 study found that 95% of women who had heart attacks started feeling some of those symptoms a month or more before.
"Women are very into their bodies -- they know when they aren't feeling well," says Nieca Goldberg, medical director of New York University's Women's Heart Program. "That's the time to call your doctor, when there's still time to prevent a heart attack."
Could some of those nonspecific chest pains in the AHRQ report have been early warning signs? There's no way of knowing, since the statistics don't track whether patients returned with a heart attack later.
Warning for Women
Chest pain is a common symptom in heart attacks, but women may instead have:
- Pressure or pain in shoulders, back, jaw or arms
- Dizziness or nausea
- Sudden fatigue or weakness
- Shortness of breath
- Unexplained anxiety
But even when women do have such diagnostic tests, doctors are less likely to find CAD. "Women with chest pain are much more likely to have normal coronary arteries," says Rita F. Redberg, director of Women's Cardiovascular Services at the University of California -- San Francisco School of Medicine. "The truth is, we don't always find out what causes it."
One theory is that some chest pain in women may be due to microvascular disease -- blockages in tiny vessels that can't be seen on an angiogram. Such blockages are also too small to be opened with stents or angioplasty, but can be treated with beta blockers and nitroglycerine.
Given the growing awareness that heart attacks present differently in women, the AHRQ data may indicate that doctors are treating women with chest pain more proactively than they used to.
"Women's chest pain is increasingly taken more seriously -- even if it's atypical -- so doctors may have a lower threshold for admitting them," says cardiologist Edmund M. Herrold, a professor of medicine at the State University of New York-Downstate Medical Center in Brooklyn. "I think that's a good thing. In the past, women who should have been admitted were being sent home."
Should women think twice about getting help if they're having chest pain? "We're always trying to draw that line fine -- not wanting people to ignore symptoms, but not thinking it's a heart attack everytime they get a twinge," says Dr. Redberg.
Studies show that women wait longer than men before going to the hospital -- in part because the symptoms may be subtle, and in part because they don't want to "bother" doctors if nothing's wrong. But two-thirds of women who have heart attacks never make it to the hospital.
If you have any of the symptoms for more than a few minutes, seek help quickly. "Don't sit home and wonder," says Dr. Goldberg, who sometimes has to beg patients who call her in the middle of the night to go to the hospital.
She also suggests calling 911 rather than going on your own: "You'll get taken care of faster in the emergency room if you arrive by ambulance. The hospital can call the doctor for you."
Beyond Hysterectomy: Alternative Options for Heavy Bleeding
By Carolyn Sayre
NY Times Article : October 3, 2008
In Brief:
Heavy or prolonged bleeding affects nearly one in five women ages 35 to 49.
Hysterectomy was the treatment of choice 20 years ago, but new options like the Mirena intrauterine device and medications often make surgery unnecessary.
Today, many women with menorrhagia continue to suffer because they are unaware of new treatment options.
It’s rare to find a woman who has never had any trouble with her period. For most, though, the hassles are fairly minor — irritability, mild cramping and the occasional surprise visit. But for the nearly one in five women ages 35 to 49 who suffer from menorrhagia — heavy or prolonged bleeding — their menstrual cycle puts life on hold every month.
“It controlled my life,” said Stefanie Haase, 37, an X-ray technician from Sodus, N.Y., who started bleeding uncontrollably two years ago because of a large fibroid. “I had to carry an extra set of clothes with me at all times. I couldn’t drive without messing up the car, or plan a vacation. My life revolved around my period.”
Twenty years ago, women like Ms. Haase did not have many options. For the nearly 40 percent of women whose abnormal bleeding stemmed from anatomical problems like fibroids and polyps, an invasive surgical procedure to remove the noncancerous growths, called a myomectomy, was often helpful but did not always stop the bleeding. For the majority of patients, the outlook was pretty unpleasant. High-dose hormones caused a host of side effects like rapid weight gain. A procedure called dilation and curettage, or D&C, which scraped away the lining of the uterus, typically failed to stop the bleeding for more than a few months.
Ms. Haase would have quickly found herself out of options and, like many women living with menorrhagia back then, her last resort would likely have been a hysterectomy — major surgery to remove the uterus and sometimes other reproductive organs as well. But thanks to new treatments and techniques, all that has changed.
“Women no longer need to choose between suffering and major surgery,” said Dr. Glenn Schattman, a reproductive surgeon and associate professor of obstetrics and gynecology at the New York Presbyterian Hospital/Weill Medical College of Cornell. “Today, the key is avoiding major surgery, and thankfully, the overwhelming majority of patients can now be treated with minimally invasive procedures or medication.”
The changes began in 1987, when the Food and Drug Administration approved a laser that could destroy the lining of the uterus by heating it. The laser outpatient procedure that became known as endometrial ablation was a retreat from the finality of a hysterectomy, which not only removes major organs but, back then, also required up to two months of recovery time.
A few years later, physicians began to refine the procedure, developing more sophisticated techniques like endometrial resection, which removes the uterine lining entirely.
“Women who lived 20 years ago were pushed onto a track with very few off-ramps,” said Dr. Morris Wortman, director of the Center for Menstrual Disorders & Reproductive Choice in Rochester. “Endometrial ablation changed that by obviating the need for hysterectomy. It gave women choices.”
Dr. Wortman notes that endometrial ablation stops bleeding in nearly half of women and significantly reduces bleeding in another 40 percent.
But like most procedures, endometrial ablation and resection also have downsides. About 15 percent of patients still need a hysterectomy, and while the uterus remains intact after the endometrial procedures, a woman’s chance of conception is destroyed.
Medical therapies now help eliminate some of the fertility concerns. In the early ’90s, low-dose birth control pills began to be prescribed for women with heavy bleeding who did not have structural problems. Perhaps the biggest breakthrough came in 2001, when the F.D.A. approved Mirena, an intrauterine birth control device that lightens bleeding in more than 80 percent of women.
Today, most physicians consider the fertility-preserving Mirena IUD to be the first-line treatment for heavy bleeding in women with normal anatomy.
“It works beautifully,” said Dr. Linda Bradley, director of hysteroscopic services for the department of obstetrics and gynecology at the Cleveland Clinic’s Center for Menstrual Disorders and Fibroids. “Women who take it either don’t get their period or bleed very lightly.”
That was certainly the case for Sheila Woodruff, 45, of Cleveland, who started using the Mirena IUD last spring. After two years of heavy bleeding that lasted up to 10 days every month and caused her to change her clothes every couple hours, she finally had enough.
The Mirena IUD, she said, “was unbelievable.”
“My period magically became normal again.”
In a small percentage of women, the device causes side effects like spotting or abdominal pain and may need to be removed.
And for women with anatomical problems like fibroids and polyps, minimally invasive procedures like laparoscopic myomectomy have reduced recovery time significantly. And uterine fibroid embolization, a nonsurgical technique that shrinks or kills the growths by cutting off their blood supply, has helped certain patients with structural problems avoid surgery altogether.
Now that alternative treatments to hysterectomy have been developed, physicians say their biggest challenge is raising awareness and acceptance among women, many of whom still fear that major surgery is their only option.
“I can’t believe how many women are essentially bedridden during their periods for a year or two before they see a doctor,” Dr. Schattman said. “They are still scared that heavy bleeding means they will need a hysterectomy.”
According to the Centers for Disease Control and Prevention, 20 million women now living in the United States have had a hysterectomy. “That number should be lower,” Dr. Wortman said. “And it would be if more women understood all of their options.”
Despite the fact that many physicians say the Mirena device is safe and remarkably effective, women who remember the infamous side effects of the Dalkon Shield, a popular IUD used in the ’70s, still shy away from using it. What’s more, techniques like endometrial ablation and resection have only really begun to catch on in the last 10 years or so as technological advancements have made it easier for less-specialized gynecologists to perform the procedures.
Ms. Haase, for one, waited it out for nearly two years before she underwent surgery. Unlike most patients with fibroids, she underwent both a myomectomy and endometrial resection to stop her bleeding.
“My mother had a hysterectomy when she was 33, so I figured that was my fate,” she said. “But there are so many options out there. I only wish I had known that two years ago.”
More doctors are challenging the convention of removing the cervix during a hysterectomy
By Laura Johannes
WSJ Article : February 24, 2009
For decades, surgeons performing a hysterectomy, or removal of the uterus, typically cut out the woman's cervix as well. If it were left in, doctors reasoned, it could develop cancer.
Now, a growing number of gynecologists are marketing "cervix-sparing" hysterectomies if cancer isn't present. The chance of cervical cancer is fairly low, and Pap-smear screening will catch most cases, these doctors say. And leaving the cervix untouched reduces the risk of surgical damage to the bladder and nearby nerves, and may even allow a woman to enjoy a better sex life long term, say doctors who perform these procedures.
Not so fast, argues the American College of Obstetricians and Gynecologists. The influential nonprofit doctors' group argued in a 2007 report that evidence was lacking that sparing the cervix improves one's sex life or bladder function. And if a growth develops, removing the cervix alone carries higher risk, including infection, than a hysterectomy, the group says. "It's not appropriate for surgeons to recommend it as a superior technique," says Denise Jamieson, chairman of the committee that wrote the report.
Cutting Less
More doctors are challenging the convention of removing the cervix during a hysterectomy. Here's why:
- Pap smears have sharply reduced the incidence of cervical cancer.
- Sparing the cervix reduces the risk of bladder damage.
- Some doctors say it may improve sexual function.
Now the tide appears to be turning. In 2006, 9.7% of U.S. inpatient hysterectomies, which account for more than 90% of the procedures, preserved the cervix, compared with just 1.7% a decade earlier, according to federal data.
The cervix is a small, doughnut-shaped mass of tissue at the base of the uterus. Downsides to keeping it include the need to continue regular Pap smears and the possibility of continued menstrual bleeding or spotting, if the surgeon doesn't succeed in removing all of the uterus.
"When you give women the choice, and you tell them the pros and cons, many of them find the idea of keeping the cervix very appealing," says Seth Kivnick, a physician at Kaiser Foundation Hospital in West Los Angeles. Three-quarters of women without cancer choose to keep the cervix when having a hysterectomy at the hospital, he says.
Some researchers believe that for at least some women, the cervix may contribute to sexual pleasure; doctors also say leaving it in place makes it easier to avoid unwittingly shortening the vaginal canal. A 212-patient Finnish study from 1983 found pain upon intercourse pre-hysterectomy was better relieved by a cervix-sparing procedure. A parallel study, involving the same women, found the frequency of orgasms decreased in women who had their cervix removed but not in those who didn't.
Dr. Jamieson, of the American College of Obstetricians and Gynecologists, says the orgasm finding didn't reach statistical significance. Also, the study designs were flawed, in part because the women weren't assigned randomly to the different procedures, she says.
Other studies have failed to validate a positive role for sparing the cervix. A 2005 study of 135 women found no difference in sexual functioning or quality of life between women who had the cervix-sparing and regular hysterectomies. "It's not to say that an individual woman may not be in a better place, but on average it's not true," says lead author Miriam Kuppermann, an epidemiologist at the University of California, San Francisco.
One limitation of the research is that various studies, including Dr. Kuppermann's, compared hysterectomies done with a large abdominal incision. Many surgeons pushing the cervix-sparing procedure do a minimally invasive version involving only three or four tiny incisions, which helps shorten recovery times. Removal of both the uterus and the cervix can also be performed minimally invasively, but this option isn't yet widely available. In another type of procedure, called "vaginal hysterectomy," no abdominal incisions are made and the uterus is removed through the vagina; the cervix generally must be removed. Women whose Pap smears recently found lesions that could be pre-cancerous are generally advised to have the cervix removed during a hysterectomy. Some physicians won't leave the cervix in place if a woman tests positive for strains of human papillomavirus that can cause cervical cancer.
A Dip in the Sex Drive, Tied to Menopause
By Jane E. Brody
NY Times Article ; March 31, 2009
Concern about the safety of hormone replacement has all but obscured one of the most pressing concerns for women of a certain age: the effects of menopause on their sex lives. Many are reluctant to ask their doctors a question uppermost in their minds: “What has happened to my desire for sex and my ability to enjoy it?”
With fully a third of their lives ahead of them, but with little or none of the hormones that fostered what may have been a robust sex life, many postmenopausal women experience diminished or absent sexual desire, difficulty becoming aroused or achieving orgasm, or pain during intercourse caused by menopause-related vaginal changes.
Sometimes the reasons for these problems go beyond hormones. Some women may consider themselves less sexually attractive as their bodies change with age, or they have partners who have lost interest in sex or the ability to perform reliably.
But for most postmenopausal women, hormone-related changes are the primary factors that interfere with sexual satisfaction. My friend Linda, for example, who lives in Pittsburgh, was 52 years old and recently married when her vibrant interest in sex suddenly plummeted, leading to a frantic search for a way to restore it.
A more common situation is described by Pat Wingart and Barbara Kantrowitz in their informative book, “Is It Hot in Here or Is It Me?” (Workman, 2006): “You’re not in the mood a lot of the time. Most nights, you just wish your partner would roll over and go to sleep. When you do feel like a little action, it takes forever to get warmed up. Sometimes sex is more painful than pleasurable.”
Common Changes
Unlike Linda, who had an abrupt change in desire, many women report a gradual decline in sexual desire as they age. In a survey of 580 menopausal women conducted by Siecus, the Sexuality Information and Education Council of the United States, 45 percent reported a decrease in sexual desire after menopause, 37 percent reported no change and 10 percent reported an increase.
Although individual experiences certainly vary, “Changes in arousal clearly are associated with menopause,” according to a 2007 article in The Journal of the American Medical Association. The author, Dr. Jennifer E. Potter of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, said physical factors include less blood flow to genital organs, a decrease in vaginal lubrication and a decreased response to touch.
Women can achieve orgasm throughout their lives, but they typically need more direct, more intense and longer stimulation of the clitoris to reach a climax, Dr. Potter noted.
Another common experience is a diminished intensity of orgasm and painful uterine contractions after orgasm, although the women surveyed by Siecus said over all that they remained satisfied with sex.
Yet as Dr. Potter put it, “What might be a satisfying sexual life for one woman may seem woefully inadequate to another,” adding that what a woman expects from her sex life can make a difference. She cited the findings of various large surveys: “Only one-third to one-half of women who report decreased desire or response believe they have a problem or feel distress for which they would like help.”
So what happens to a woman’s body when levels of sex hormones fall?
Although estrogen is a woman’s predominant hormone before menopause, testosterone, produced in women primarily by the ovaries and adrenal glands, is considered the libido hormone for both men and women.
Testosterone levels in women decline by about 50 percent between the ages of 20 and 45, and the amount of testosterone produced continues to decline gradually as women age. While menopause itself has no direct effect on testosterone production, surgical removal of the ovaries can cause an abrupt drop in this hormone and accompanying sexual desire, especially for women who have not gone through natural menopause.
For some women, the increased ratio of testosterone to estrogen that occurs after menopause gives their sex drive a boost, Ms. Wingart and Ms. Kantrowitz point out.
But for most women, the menopausal effects of low levels of estrogen are the primary deterrents to sexual pleasure. In addition to the infamous hot flashes, changes in the vagina and vulva can have serious effects on the sexual experience.
¶With little or no estrogen, vaginal walls become dry, thin and less elastic, causing pain during penetration.
¶Diminished blood flow to the genital area means it can take much longer for a woman to feel aroused.
¶The anticipation of painful uterine contractions with orgasm can be a turnoff.
¶A leakage of urine some women experience during sex can prompt them to avoid it.
Helpful Treatments
Linda, who asked that her last name not be used, said she was more concerned about reviving her sex life than a possible increased risk of hormone-induced cancer or heart disease. A prescription of the drug Estratest, which combines estrogen and testosterone, solved her problem.
But taking estrogen orally is not recommended for women who have had breast cancer or are at high risk for developing it. Also, to protect the uterus against cancer, estrogen should be combined with a progestin.
An alternative that works for some is vaginal application of a little estrogen via a cream, ring or tablet, which keeps the hormone from passing through the liver and diminishes the amount that enters the bloodstream.
Gynecologists concerned about safety are more likely to recommend a non-oil-based lubricant. Besides such popular products as K-Y jelly, Ms. Wingart and Ms. Kantrowitz suggest several longer-lasting products that have an adhesive quality, including Replens, K-Y Long-Lasting Vaginal Moisturizer and Astroglide Silken Secret. The authors said “women who have intercourse regularly seem to generate more lubrication than those who do it less frequently.”
Infrequent intercourse or prolonged periods without it can result in a narrowing of the vagina that can be countered by the use of lubricated vaginal dilators. For women whose sex lives are disrupted by lack of a partner, the authors recommend self-stimulation. Dr. Potter suggested that even for women with partners, a vibrator or small battery-powered vacuum pump can aid in arousal.
While a Viagra-like drug is not yet an option for women, use of the antidepressant bupropion (Wellbutrin at 300 milligrams a day) may improve sexual arousal and satisfaction in women who are not depressed. And Dr. Potter pointed out that remaining physically fit can also help.
The Estrogen Dilemma
By Cynthia Gorney: April 12, 2010 : NY Times Article
Here we are, two fast-talking women on estrogen, staring at a wall of live mitochondria from the brain of a rat. Mitochondria are cellular energy generators of unfathomably tiny size, but these are vivid and big because they were hit with dye in a petri dish and enlarged for projection purposes. They’re winking and zooming, like shooting stars. “Oh, my God,” Roberta Diaz Brinton said. “Look at that one. I love these. I love shooting mitochondria.”
Brinton is a brain scientist. Estrogen, particularly in its relationship to the health of the brain, is her obsession. At present it is mine too, but for more selfish reasons. We’re inside a darkened lab room in a research facility at the University of Southern California, where Brinton works. We are both in our 50s. I use estrogen, by means of a small oval patch that adheres to my skin, because of something that began happening to me nine years ago — to my brain, as a matter of fact. Brinton uses estrogen and spends her work hours experimenting with it because of her own brain and also that of a woman whose name, Brinton will say, was Dr. A. She’s dead now, this Dr. A. But during the closing years of her life she had psychologists. “We’d spend hours, me listening to her stories, and I’d walk out of the room,” Brinton told me. “Thirty seconds later, I’d walk back in. I’d say, ‘Dr. A., do you remember me?’ And she was so lovely. She’d say: ‘I’m so sorry. Should I?’ ”
The problem with the estrogen question in the year 2010 is that you set out one day to ask it in what sounds like a straightforward way — Yes or no? Do I or do I not go on sticking these patches on my back? Is hormone replacement as dangerous in the long term as people say it is? — and before long, warring medical articles are piling up, researchers are raising their voices and gesticulating excitedly and eventually you’re in Los Angeles staring at a fluorescent rodent brain in the dark. “You want a statistic?” Brinton asked softly. Something about the shooting mitochondria has made us reverent. “Sixty-eight percent of all victims of Alzheimer’s are women. Is it just because they live longer? Let’s say it is, for purposes of discussion. Let’s say it’s just because these ladies get old. Do we just say, ‘Who cares?’ and move them into a nursing home? Or alternatively, maybe they are telling us something.”
With their brains, she means. Their sputtering, fading Alzheimer’s brains, which a few decades earlier were maybe healthy brains that might have been protected from eventual damage if those women had taken estrogen, and taken it before they were long past their menopause, while their own neural matter still looked as vigorous as those rat cells on the wall. This proposition, that estrogen’s effects on our minds and our bodies may depend heavily upon when we first start taking it, is a controversial and very big idea. It has a working nickname: “the timing hypothesis.” Alzheimer’s is only one part of it. Because the timing hypothesis adds another layer of complication to the current conventional wisdom on hormone replacement, it has implications for heart disease, bone disease and the way all of us women now under 60 or so — the whole junior half of the baby boomers, that is, and all our younger sisters — could end up re-examining, again, everything the last decade was supposed to have taught us about the wisdom of taking hormones.
I first met Brinton at a scientific symposium at Stanford University in January that was entirely devoted to the timing hypothesis. The meeting was called Window of Opportunity of Estrogen Therapy for Neuroprotection, and it drew research scientists and physicians from all over the country. When I asked to listen in, the organizers hesitated; these are colleagues around a conference table, they pointed out. They’re probing, interrogating, poking holes in one another’s work in progress.
But I was finally permitted to take a chair in a corner, and as the day went on, I became aware of my patch, in a distracted, hallucinatory sort of way, as if I had started fixating on a smallish scar. One after another, their notes and empty coffee cups piling up around them, heart experts and brain experts and mood experts got up to talk about estrogen — experiments, clashing data, suppositions, mysteries. There are new hormone trials under way that are aimed at the 40-year-old to 60-year-old cohort, with first results due in 2012 and 2013. There are depression studies involving estrogen. There are dementia studies involving estrogen. There are menopausal lab monkeys taking estrogen, ovariectomized lab mice taking estrogen and young volunteers undergoing pharmaceutically induced menopause so researchers at the National Institutes of Health can study exactly what happens when the women’s estrogen and progesterone are then cranked back up. I typed notes into my laptop for hours, imagining the patch easing its molecules into the skin of my back, and the whole time I was typing, working hard to follow the large estrogen-replacement thoughts of the scientists around the table, I had one small but persistent estrogen-replacement thought of my own: If I make the wrong decision about this, I am so screwed.
I started taking estrogen because I was under the impression that I was going crazy, which turns out to be not as unusual a reaction to midlife hormonal upheaval as I thought. This was in 2001. The year is significant, because the prevailing belief about hormone replacement in 2001 was still, as it had been for a quarter century, the distillation of extensive medical and pharmaceutical-company instruction: that once women start losing estrogen, taking replacement hormones protects against heart disease, cures hot flashes, keeps the bones strong, has happy effects on the skin and sex life and carries a breast-cancer risk that’s worth considering but not worrying about too much, absent some personal history of breast cancer or a history of breast cancer in the immediate family.
At first, as I was trying to locate a psychiatrist who would take me on, I wasn’t aware I had reason to pay attention to advice about hormones at all. That year I turned 47, a normal age for beginning the drawn-out hormonal-confusion period called perimenopause, but I had none of the familiar signs. Menopausal holdouts run in the family; one of my grandmothers was nearly 60 by the time hers finally kicked in. My only problem was a new tendency to wake up some mornings with a great dark weight shoving my shoulders toward the floor and causing me to weep inside my car and basically haul myself around as if it were the world’s biggest effort to stand up straight and carry on a conversation. Except for its having shown up so arbitrarily and then coming and going in waves, there was nothing interesting about my version of what my husband and I came to think of as the Pit; anybody who has been through a depression knows what a stretch of semidisabling despair feels like, and for my part I had a very nice life, a terrific family and a personal interior chorus of quarreling voices demanding to know why I didn’t pull up my socks and carry on, which in fact was the first question I planned to ask a psychiatrist.
But I went to my gynecologist first, so she could check my blood pressure or whatever seemed the prepsychiatrist thing to do. How often would you say you feel this way, she asked; and I said I didn’t know, maybe every few weeks; and she told me to start keeping records. Note each day, she said. Check for patterns.
She was right. There was a pattern. I was falling into the Pit on schedule, around 11 days before each menstrual period, or M.P., which is one of many abbreviations I was to learn in my efforts to keep track of the ferocious hormones debate that started up in North America in 2002, one year after I stuck on the first estrogen patch that my gynecologist prescribed. The study at the center of the ruckus was called the Women’s Health Initiative, or W.H.I. It was a federally financed examination of adult women’s health, extraordinary in scale and ambition, that started up in the early 1990s; one of its drug trials enrolled more than 16,000 women for a multiyear comparison of hormone pills versus placebos. On July 9, 2002, W.H.I. investigators announced that they had ended the trial three years early, because they were persuaded that it was dangerous to the hormone-taking participants to let them continue.
The women on hormones were having more heart trouble than their placebo-taking counterparts, the investigators said, not less. Their risk for stroke went up. Their risk for blood clots went up. Their risk for breast cancer increased by 24 percent. The W.H.I. bulletins dominated medical news all summer and long into the fall, and so alarming were their broad-scale warnings that millions of women, myself included, gave up hormone replacement and resolved to forge ahead without it.
The patches my gynecologist prescribed worked, by the way. I didn’t understand how, beyond the evident quieting of some vicious recurring hormonal hiccup, and neither did the gynecologist. But she had other women who came in sounding like me and then felt better on estrogen, and I would guess many of them, too, decided after the W.H.I. news that they could surely find other ways to manage their “mood swings,” to use the wondrously bland phrasing of the medical texts. (I’m sorry, but only someone who has never experienced one could describe a day of “I would stab everyone I know with a fork if only I could stop weeping long enough to get out of this car” as a “mood swing.”) We muddled along patchless, my mood swings and my patient family and I, until there came a time in 2006 when in the midst of some work stress, intense but not unfamiliar, I found myself in a particularly bad Pit episode and this time unable to pull out.
It was profoundly scary. In retrospect, I managed a surprising level of public discretion about what was going on; competence at the cover act is a skill commonly acquired by midlife women, I think, especially those with children and work lives. If the years have taught us nothing else, they have taught us how to do a half dozen things at once, at least a couple of them decently well. Like other women I have met recently with stories like this one, I relied for a few months on locked office doors, emergency midday face-washings and frequent visits to an increasingly concerned talk therapist. But one afternoon I got off my bicycle in the middle of a ride with my husband, because I had been crying so hard that I couldn’t see the lane lines, and I sat down on the sidewalk and told him how much I had come to hate knowing that family obligations meant I wasn’t allowed to end my life. The urgent-care people at my health clinic arranged a psychiatric consult fast, and after listening and nodding and grabbing scratch paper to draw me an explanatory graph with overlapping lines that peaked and plunged, the psychiatrist wrote me two prescriptions. One was for an antidepressant.
The other — I recognized the name as soon as she wrote it down — was for Climara, my old estrogen patch.
By this time we were four years past the 2002 W.H.I. hormone news. So I knew a few more things. I knew there had been a surge of industrious scrambling among former hormone-taking women, some of whom had tried multiple alternatives or going cold turkey and then changed their minds and re-upped on estrogen, deciding that life without it was so unpleasant that they no longer cared what the statistical prognoses said. I knew the prevailing medical sentiment had shifted slightly since the bombshell of 2002; certain articles and books still urged women to shun hormone replacement at all costs, but the more typical revised counsel was, essentially, proceed with great caution. If some menopausal malady is genuinely making you miserable, the new conventional wisdom advised, and no alternative remedy is working for you, then go ahead and take hormones — but keep the dose low and stop them as soon as possible.
I would like to be able to tell you that I weighed these matters thoughtfully, comparing my risks and benefits and bearing in mind the daunting influence of a drug industry that stands to profit handsomely from the medicalizing of normal female aging. But that would be nonsense, of course. I was too crazy. I went straight to the pharmacy and took everything they gave me.
You don’t read the fine print on package labels when you’re being ushered through a psychiatric crisis, but after a while, I did. By last winter I was nearing the cumulative five-year mark as an estrogen user, and although “low dose, stop soon” is often an advisory without specifics attached, five years seemed to turn up here and there as an informal outer-limit guideline. And because it had worked again, because the estrogen so clearly helped repair something that was breaking (there’s no way for me to separate the effects of estrogen from the effects of the antidepressant, except that on the few occasions when I’ve been haphazard about replacing the estrogen patches on time, I’ve experienced prompt and unmistakable intimations of oncoming Pit), I now had some rational faculties with which to go looking for explanations that might help me decide what to do. This was when I first began learning that in the controversy over hormone replacement, the fine print matters a very great deal.
First of all, the kind of estrogen in my patches — there are different forms of estrogenic molecules — is called estradiol. It’s not the estrogen used in the W.H.I. study. Pharmaceutical estradiol like mine comes from plants whose molecules have been tweaked in labs until they are atom for atom identical to human estradiol, the most prominent of the estrogens premenopausal women produce naturally on their own. The W.H.I. estrogen, by contrast, was a concentrated soup of a pill that is manufactured from the urine of pregnant mares. The drug company Wyeth (now owned by Pfizer) sells it in two patented products, the pills Premarin and Prempro, and it’s commonly referred to as “conjugated equine estrogens.”
There was more in the fine print. Two years ago, after warning me that women who haven’t had a hysterectomy run a higher risk of uterine cancer when they take only estrogen as hormone replacement, a new doctor added in progesterone, which has been shown to protect the uterus. The progesterone he prescribed for me, like the estradiol, is a molecular replica of the progesterone women make naturally. It’s different from the progesteronelike synthetic hormone that was used for the W.H.I. study that ended in 2002. That medication was a formulation whose multisyllabic chemical name shortens to MPA and which has a problematic back story of its own: MPA takes care of the uterine-cancer risk, but there’s reason to suspect it may be a factor in promoting breast cancer. And it’s ingested as a pill, which means that like equine estrogens (and unlike, for example, my patch), MPA metabolizes through the liver, possibly creating additional complications en route, before going about its business.
The biggest difference between me and the W.H.I. women, though, has to do with age and timing. I started on the patches while my own estrogen, pernicious though its spikes and plummets may have been, was still floating around at more or less full strength. The average age of the W.H.I. women was just over 63, though the study accepted women as young as 50. More significant, though, most of them were many years past their final menstrual period, which is the technical definition of menopause, when they began their trial hormones. The bulk of the group was at least 10 years past; factoring in the oldest women, the average number of years between the volunteers’ menopause and their start on the trial medications was 13.4.
Because women generally make decisions about hormones while they are in the throes of perimenopause — that term is now used to extend through the year following the final M.P. — you may find this as perplexing as I did. Why would the largest drug trial in the history of women’s health select, for most of its participants, women already long past the critical phase? I heard one undiplomatic critic sum up the W.H.I. as “the wrong drugs, tested on the wrong population,” and those two factors, the drugs and the population, are actually directly linked. Equine estrogens and MPA were the only forms of hormones used in the W.H.I. trials. Among other reasons, that’s because drug trials are expensive; this one was huge, and Wyeth was going to provide without cost an average of eight years’ worth of its equine estrogens and MPA to 40 clinical centers.
And millions of women were using those very hormones already, partly because aggressive Wyeth marketing had for three decades insisted that hormone replacement was the ticket to a vigorous and sexually satisfactory postmenopausal life. To a certain extent, evidence backed up that claim; wide-scale though less rigorous earlier studies appeared to demonstrate hormone replacement’s benefits so clearly that many physicians were suggesting it almost automatically to midlife women, whether or not they had perimenopausal complaints. Hormones raised the breast-cancer risk in those earlier studies, but nearly every other health factor showed improvement when women who took hormones were compared with those who didn’t. Hot flashes disappeared, osteoporosis was milder, women reported feeling better and women who took hormones showed a markedly lower rate of heart disease than women who did not.
Because heart disease ultimately kills many more women than all cancers combined, some doctors had also taken to urging older women, even those past menopause, to start hormones for cardiac-health purposes. The W.H.I. trials were supposed to provide conclusive evidence, finally, as to whether all this wide-scale prescribing was truly a sound idea. But cardiovascular disease tends to make its bids for attention — its “events,” as clinicians say, like heart attack and death — when we’re quite a bit past 51, the average age at which American women hit menopause. The only way the W.H.I. was going to tally up a scientifically useful number of cardiac events was to enroll plenty of women already old enough to reach that danger stage before the study’s time ran out. So that’s what they did, and once the final data was reparsed many times, it was clear that the trial had shown physicians something highly important about the perils of starting older postmenopausal women (that’s qualifier No. 1) on pills (No. 2) containing equine estrogens (No. 3) plus MPA (No. 4).
Those four qualifiers make the chief message of the W.H.I. — that taking hormones, in the long run, is more likely to hurt you than help — far more specific than the one most women heard. For those of us not yet on the far side of menopause, or who don’t match the other qualifiers (as I write this, for example, I’m zero for four), a daunting proportion of what we thought we learned about hormone replacement over the last eight years remains unsettled, more confusing than ever and conceivably — we don’t know yet — wrong. “I mean, if you’re a 70-year-old,” says S. Mitchell Harman, a Phoenix-based endocrinologist and coordinator of one of the national trials currently examining hormones’ effects on younger women, “and your question is, Should I start taking estrogen? the W.H.I. answered that for you beautifully. No. Unfortunately, it wasn’t designed to answer that question for a 50-year-old. So now we’re trying to fill in the blanks.”
One afternoon last month, I reported to the Northern California site for an N.I.H.-financed cognitive trial that is part of the Kronos Early Estrogen Prevention Study that Harman is leading. Keeps, as it’s called, has enrolled women at nine such sites around the country; this one was inside a medical building at the University of California, San Francisco, and the cognition test I asked to try proved to be a low-tech experience: a table with chairs, pens and pencils and a gentle-voiced psychologist asking me to do things with my brain. Number sequences repeated backward, lists of random objects to recall, designs to remember and copy — I promised not to describe specifics, because making details public could compromise the trial results. But imagine a stranger holding up a stopwatch and giving you 30 seconds to name every dessert item you can think of. The brain charges off into a comical panic grope, and it’s like a cross between a back-seat car game and the SATs.
The only grading marker, though, is self compared to self. If I were a Keeps participant, I would be on a four-year regimen of some mystery medication — either estrogen, in one of two forms (estradiol patches or equine-estrogen pills, to see whether differences emerge between the two), or placebo patches or placebo pills. Then in another year, I would retake the cognition test, which lasted about an hour and a half, so researchers could track any change. Brain function is a major element of the Keeps agenda; the other is heart health, so the test administrators would conduct annual ultrasounds of my carotid artery, to check for the thickening that signals heart disease. That’s how they are trying to circumvent the doesn’t-manifest-until-you’re-older problem, by measuring for known warning markers rather than waiting for the actual big events. They would check my blood and cholesterol for signs of other cardiovascular trouble.
With about 730 participants, Keeps is relatively small; hormone research has been tough to finance in the post-W.H.I. years, and every scientist and physician I’ve spoken to said there will never again be another hormone trial as costly and ambitious as the W.H.I. A second study, based in Los Angeles, called the Early Versus Late Intervention Trial With Estradiol, is following more than 600 women — comparing a group that has been post-menopausal for an average of 15 years and that is on estradiol or on a placebo with a second, younger group that is an average of three years post-menopausal. “This is the age when we should really study estrogen,” says Sanjay Asthana, a University of Wisconsin medical professor who is a designer of the cognition component of Keeps. “People like me are really waiting to see what this data looks like. Either way. We need to know.”
Asthana is a geriatrician, with a specialty in Alzheimer’s and other forms of age-related memory loss. That makes him a member of what I came to think of, in my travels among estrogen researchers this winter, as the brain contingent. Their working material includes neuroimaging; magnified slices of rodent brains; and live cells that carry on in petri dishes, shooting mitochondria around or struggling under the burden of disease. All these things allow the brain contingent to see, sometimes literally, estrogen in action. It’s an amazing process. When cells are healthy, estrogenic molecules slide right in, searching for special receptors that are shaped precisely for the estrogens: the receptors are tiny locks, waiting for the right molecular keys to turn them on. Then, once they are activated by the key-turning process, the work estrogen receptors do is richly complex, if only partly understood. They prod genes into action; they raise good cholesterol; they affect the neurotransmitter chemicals associated with mood and stress, like serotonin and dopamine.
And the brain, scientists have learned in recent decades, is loaded with these receptors. Knowing this makes it easier to understand how perimenopause could start inside aging ovaries and set off such a wild cascade of effects. If you’re a typical woman moving through your 40s or 50s, your lifetime egg supply is running out; as that happens, the intricate, multihormone reproductive-signaling loop grows confounded, its triggers altered by the biology of change. The brain and ovaries, the primary stops along this loop, start misreading each other’s demands for action. This can make estrogen production crank up frantically, crash and then crank up again. Something also goes awry with most women’s thermoregulatory systems, producing hot flashes in around three-quarters of us — nobody yet knows why, exactly, nor why certain women go on flashing for many years while some escape the whole must-remove-outer-garments-now
Not all women, Manson notes, experience disruptions as robust as this unidentified patient’s. But consider the mess of internal rearrangement we’re looking at: the body’s overall estrogen production is waning as the ovaries start atrophying into full retirement; and here simultaneously, at least for some of us, is this great Upheaval of During. The combination of the two can be — how could it not, I thought, the first time I studied the three graphs — a hellacious strain on the brain. Tracing the exact mechanics is still a work in progress, but they surely include some disruption of signaling to the neurotransmitters that make us remember things, experience emotions and generally choreograph the whole thinking operation of the human self.
“There are all these fundamental cognitive functions that many perimenopausal women complain about, and one of those fundamentals is attention,” Roberta Brinton, the U.S.C. scientist, told me. “When you can’t hold your attention to a thought. Where you’re in constant start mode, and you never reach the finish mode. That is devastating.”
This was Brinton, as it happens, describing herself. It’s why she first went on estrogen (estradiol, accompanied by natural progesterone) when her own perimenopause kicked in a few years ago. We were sitting in a campus garage in her Prius one day, and I asked her what made her so sure her own midlife difficulties — she had the hot flashes, which were obvious, but also the sleep disruption and the infuriating distractibility — were the product of hormonal events, not some womanly existential crisis. We get a lot of that, societally. It’s meant to be empathetic. Your role in life is changing, Mrs. Brain Seized by Aliens! Your children are growing up, you’re buying expensive wrinkle cream, ice cream makes you gain weight now, of course you’re distraught! “Because with estrogen — ” Brinton looked at me sharply, and then smiled — “I don’t have attention-deficit disorder.”
We walked back up to her laboratories, which are spread along a many-roomed warren full of cell incubators, centrifuges and computers. Brinton has thick black hair and a demeanor of lively, good-humored authority; it’s easy to envision her as the passionate science professor in crowded lecture halls. But in her labs the work is all rats and mice, many of them surgically or genetically altered to serve as surrogates for adult humans in various stages of maturation or disease. Removing the ovaries from female rats, for example, sends them into low-estrogen mode. Mice can be ordered bred with Alzheimer’s. The plaque that clogs the brains of Alzheimer’s sufferers, a noxious memory-disrupting substance called beta amyloid, is available as a chemical distillate, which means Brinton’s team can experiment with that too — beta amyloid dropped into the brain cells of healthy low-estrogen rodents; or estrogen dropped into cells already damaged by beta amyloid.
That’s why Brinton says that the timing hypothesis — the proposition that estrogen could bring great benefit to a woman who starts it in her 50s while having the reverse effect on a woman 10 years older — makes sense even though it is still experimental. She and other scientists know there are ways estrogen improves and protects the brain when it is added to healthy tissue. It makes new cells grow. It increases what’s called “plasticity,” the brain’s ability to change and respond to stimulation. It builds up the density and number of dendritic spines, the barbs that stick out along the long tails of brain cells, like thorns on a blackberry stem, and hook up with other neurons to transmit information back and forth. (The thinning of those spines is a classic sign of Alzheimer’s.)
But when estrogen hits cells that are already sick — because they’re dying off as part of the natural aging process or because they’ve been damaged by beta amyloid — something else seems to happen. Dropped in as a new agent, like the wrong kind of chemical solvent sloshed onto rusting metal, estrogen doesn’t strengthen or repair. It appears useless. Sometimes it sets off discernible harm. You may recall additional W.H.I. news a few years ago about hormones increasing the risk for aging-related dementia; those stories emerged from a subgroup of W.H.I. participants who were all at least 65 when they started the hormones. There are arguments about that data, like nearly everything else connected to the W.H.I., but the age factor alone reinforces what Brinton and other timing-hypothesis researchers observe in the labs when they give estrogen to ailing cells. “It’s like the estrogen is egging on the negative now, rather than the positive,” she said. “We know that if you give neurons estrogen, and then expose them to beta amyloid, many more will survive. But when we expose them to amyloid and then give them estrogen — now you don’t have survival of the neurons. In some instances, you can actually exacerbate their death.”
The heart contingent exploring the timing hypothesis is reasoning the same way. Monkeys get both cardiovascular disease and their own version of menopause; there is a primate team at Wake Forest University in North Carolina that has found estrogen to be a strong protectant for females against future heart disease — but only when it’s given at monkey perimenopause. Give estrogen the equivalent of six human years later, says Tom Clarkson, the pathology professor who has been leading this work for decades, and there is no protective effect at all.
Clarkson, who is 78, told me that if he were 30 years younger and a woman, with hot flashes or sleep trouble or sudden crashes of mood, he would have no hesitation about taking hormones. “I absolutely believe in the timing hypothesis,” he said. Then, being a scientist, he corrected himself. “I would have to say my level of certainty is 95 percent or greater,” he said. “I live a life of believing in the experimental evidence.”
So noted, I replied. And what if the symptoms were annoying but bearable or there were no symptoms at all? I’ve asked the same questions to every researcher I talked to this spring, and nearly all of them reply the same way: if they were deciding for themselves personally, they would tip the risk-benefit scale strongly in favor of hormones as a remedy for immediate ailments of perimenopause. But estrogen solely as a protectant for the heart and brain, to be taken for many years, absent any immediate serious complaints? There was a pause, and I heard Clarkson sigh. “We just don’t know about that yet,” he said.
The personal calculus of risk is an exhausting exercise in the modern era, what with litigation-jumpy physicians, the researchers’ candid “We just don’t know” and the bottomless learn-it-yourself maw of the Internet. Of all the conversations I had this winter, as I weighed and reweighed the stopping of the patch, the one that most resonates took place on a snowy morning in Washington, in the office of a nursery-school director named Julia Berry. Berry lives not far from the headquarters of the National Institutes of Health in Bethesda, Md., which is why last September she pulled from her mailbox a card the N.I.H. has been mailing to local women within a certain age range. “If you struggle with irritability, anxiety, sadness or loss of enjoyment at the time of the menopausal transition,” the card reads, “please call us and help yourself while helping others.”
The N.I.H., it turns out, has been quietly conducting mood and hormone studies for more than two decades under the direction of a psychiatrist named Peter Schmidt and his predecessor, David Rubinow, who is now chairman of the psychiatry department at the University of North Carolina. The research was first set into motion by Rubinow’s postgraduate interest in premenstrual syndrome; the idea of giving younger women drugs to lower and flatten temporarily their estrogen and progesterone levels, essentially inducing menopause, was initially conceived to determine the role of hormones in PMS — to see whether these young women got relief when their hormones stopped the cresting and dropping of the normal menstrual cycle. (It often worked as a short-term treatment and yes, the young women often got hot flashes.) In recent years, the induced-menopause experiments have continued, among many other studies, as part of an effort to try to understand the chemistry of women like Julia Berry and me — women for whom perimenopause turns into what Berry described to me as “psychological misery, not myself and absent from the world.”
Berry is 55, ponytailed and roundish and pretty. She was divorced a long time ago, raised three good kids mostly on her own and has a firm handshake and a job she loves. Her troubles started in her late 40s, in the standard way, with hot flashes and jerking awake at 3 a.m. and then escalated into something much fiercer. Like me, at the worst of it, she occasionally found herself in traffic, wishing silently for an oncoming truck that might exit her swiftly from this life without qualifying as a suicide. A physician prescribed antidepressants. They helped, with both the anguish and the flashes, but not enough. “I am one of the most steady, even-keeled, hard to ruffle, really unflappable . . . truly,” Berry told me. “I am. I, generally speaking, can be completely relied upon to do the sensible right thing almost all the time. Which is one of the reasons this period in my life has been so weird.”
She called the N.I.H. number at once. She was quickly evaluated, enrolled in a double-blind study of the effects of estrogen on perimenopausal depression and sent home with a paper bag containing a mystery patch. When I asked Berry to describe the sensation of the next few weeks, she looked up at the ceiling for a second to think. “Kind of like having been in a smoky room, waving your arms and now seeing that the exhaust fan is taking a little at a time,” she said. “My mood lifted. First time in three years I wasn’t waking up at 3 in the morning. That’s when I knew I wasn’t on the placebo. It was very clear to me that there was something fundamentally wrong with my chemical systems, and that whatever was in this patch was setting things right, so that I could function like a regular human being — the human being I was familiar with.”
What medicine doesn’t know about the chemistry of mood, including clinical depression, dwarfs what medicine doesn’t know about hormones. It would be handy for science if Berry and I could have made our heads available for dissection at certain points in recent years; as it is, we’re able to answer as many elaborations on “I feel bad” or “I feel good” as researchers might wish to throw at us, but they still have no way of pinning down where we belong on the scale of menopausal distress, or what exactly we’re doing there. We could be extra-high-volume versions of the women who are having an ordinary rough time of it, like Roberta Brinton — the women who hot-flash and can’t sleep and cast about for vocabulary with which to describe feeling, as Brinton puts it, “just off.” Or we could belong to some subcategory of anomalies, women with a wired-in susceptibility to depression — gene pools, childhoods, whatever — that was fired up by abrupt hormonal change.
Some psychological surveys will tell you there’s no evidence for a surge of clinical depression at menopause. I believe that, given how many other phases of life can unhinge us, but I also believe — no, actually, I know — that there is a difficult thing that happens to some women in the perimenopausally affected brain. Hostile as I am to generalizations involving women rendered fragile by biology, here I am, and here, too, is Berry, both of us pulled out of something terrible by a pharmaceutical infusion of estrogen. Two physicians who specialize in hormones and mood, Louann Brizendine, a neuropsychiatrist at the University of California, San Francisco, and Claudio Soares, a Canadian research psychiatrist who works at McMaster University in Ontario, told me that women who seek them out tell variations of the same story Berry and I took to our doctors: I know that something is wrong with me because I also know, somewhere in the noncrazy part of myself, that there is such pleasure to be offered by the circumstances of my grown-up life.
“These women thought they were losing their minds,” Brizendine told me, describing the 40-to-60-year-old patients she began seeing when she opened the Women’s Mood and Hormone Clinic at the university in 1994. “In 1994 we didn’t have words for it,” she said. “Now we do. It’s called perimenopausal depression.”
Brizendine and Soares, like Schmidt and Rubinow, have found that various combinations work with varying degrees of effectiveness for many of us — hormones with an antidepressant, hormones without an antidepressant, sometimes antidepressants on their own. The alternatives-to-hormones recommendations are mostly fine things in their own right, varying from certainly useful to harmless: exercise regularly, keep the weight down, easy on the caffeine, calm yourself with deep breathing or yoga, try black cohosh. (You could start a bar brawl over the efficacy of black cohosh, but the general consensus seems to be: if it works for you, go for it.) But the troubles set off by ricocheting hormones are reliably fixed by making the hormones stop ricocheting. And the laborious weighing of hormones’ benefits versus hormones’ harms — maybe not at the crisis moment, for those of us at our most distraught, but later, one or two or five years down the road — is something still undertaken by millions of women along the full breadth of the perimenopausal spectrum.
How in the world to do it wisely enough so the calculation is as right for each of us as it can possibly be? JoAnn Manson’s book contains the most careful checklist I’ve seen yet; by the time you answer all the personal-history questions the book asks you to consider, you’ve read 82 pages. Breast cancer is a factor, to be sure, but so are colorectal cancer, ovarian cancer, stroke, hip fracture and diabetes. If the timing hypothesis proves right and estrogen really does protect our brains and our hearts as long as we start it early enough, the calculation only grows that much more important and complex. There are moving pieces involved in working out every one of these risks in relation to everything else, and anyone who thinks there’s a bumper-sticker answer to the hormones question — don’t take them, you’re sure to be better off — is, like me that day in the psych unit, neither listening to scientific argument nor reading the fine print.
Here’s one example from the many to which researchers have pointed me this winter. Remember MPA? The synthetic progesteronelike substance used along with equine estrogens in the W.H.I.? There was a second W.H.I. hormones-versus-placebo trial, of nearly 11,000 women, that was also started in the early 1990s, just like the one that was halted in 2002. All the women enrolled in this second study had undergone hysterectomies, which meant they had zero risk for uterine cancer. So the women on medications in this trial were taking only equine estrogens — no MPA, which you’ll recall is given to protect the uterus. Their study was stopped in 2004, also before its planned end date, because the estrogen-taking women were showing a higher risk of stroke than the women on the placebo. But their breast-cancer rate was lower. The hormone-taking women with hysterectomies in that second study, who used estrogen without MPA, showed a 23 percent lower risk of invasive breast cancer than their counterparts who were taking no hormones at all.
Nobody’s persuaded that this means MPA promotes breast cancer while estrogen does not. It’s clear that estrogen acts aggressively on certain breast malignancies and that any woman who has had breast cancer or has a history of it in her immediate family should stay off estrogen. This is one of the principal reasons such intense work is under way right now, in labs like Roberta Brinton’s, to develop estrogenic variants — molecular substances designed to latch only to certain receptors (in the brain, say, where the activated receptors can do their good works) while ignoring receptors in the breast and uterus. And there are plenty of confounding factors, as scientists say, with regard to the women in the no-MPA trial. They all had undergone hysterectomies, for one thing; maybe whatever caused them to require uterine removal in the first place affected their reactions to the estrogen.
Or it could have been a fluke. But the MPA wrinkle adds suspicion and urgency to the timing-hypothesis questions about what really goes on when women of our demographic use hormones, and Julia Berry and I spent a long time talking about this, the adding and subtracting, the guessing and weighing, the balancing of what we think we know about ourselves against what we cannot possibly foresee. We will both, for the present, continue wearing estrogen patches. Berry turned out to be right, of course; she wasn’t on the placebo, which the N.I.H. doctors told her when she finished the study. And as she hurried to fill her own patch prescription, she found her gratitude mixed with more than a little frustration. “Why did my primary-care physician give me an antidepressant when I could have had something simple, like estrogen?” she asked. “Why don’t they know?”
We talked about breast cancer, because that is the nightmare illness in nearly all our calculations, for most of us the visual closest to hand. Three of my best friends have endured the full breast-cancer horror show and by now have retired their wigs. All have survived. None had been on hormone replacement. This is information that batters me steadily but not helpfully, like my ex-smoker paternal aunt’s fatal lung cancer and the fact that I’m a lifetime nonsmoker and regular exerciser with extremely good cholesterol levels. How do my lowered risks from one column balance against my question marks over in another column? What to do?
“I’d rather monitor something I know can go wrong than go on living in the state I was in,” Berry said. “I could have my breasts removed. I like them. But they’re not my life.”
We’ve spent a fair bit of time by now, Julia Berry and I, shaking these uncertainties out and squinting at them. Do we wear these patches forever? We don’t know. What happens when we do take them off, if we do? We don’t know. Have we done nothing except delay a biological process, complete with hot flashes and another round of truck-crash fantasies, that at some point we’ll have to bully our way through? We don’t know, nor does any researcher I talked to this spring.
And there’s this: Should luck and longevity cooperate, we are going to grow old. We’re already old, by the standards of our children and our ancestors, but the generation to which we belong expects to live a rich messy life full of extremely loud rock music for another 30 years after menopause. Every midlife woman I know keeps redrawing for herself the defensible lines of intervention in the “natural” sequence of human aging. Obsessive multiple plastic surgeries are silly and desperate. Muscles kept in good working order are not. Where on that spectrum is a hormones-saturated pharmaceutical patch? What if the timing hypothesis is even partly right? Suppose all we learn about replacement estrogen, in the end, is that if it’s started early enough it might protect the heart and the brain, and that its chemistry makes some of us feel more the way we did at 40 than the way our mothers did at 65? Not an elixir of youth. More like . . . reading glasses. Or calcium supplements, or painkillers that stop the knee from hurting but carry risk warnings of their own. It has occurred to me that the better analogy might be a 13-year-old trying to ward off puberty by binding her breasts, but most of the time I don’t think so, and if I do try stopping the patches, I know this to a certainty: I will keep a few extras in reserve, just in case.
Cynthia Gorney is a contributing writer to the magazine. She teaches at the Graduate School of Journalism at the University of California, Berkeley.
By Cynthia Gorney: April 12, 2010 : NY Times Article
Here we are, two fast-talking women on estrogen, staring at a wall of live mitochondria from the brain of a rat. Mitochondria are cellular energy generators of unfathomably tiny size, but these are vivid and big because they were hit with dye in a petri dish and enlarged for projection purposes. They’re winking and zooming, like shooting stars. “Oh, my God,” Roberta Diaz Brinton said. “Look at that one. I love these. I love shooting mitochondria.”
Brinton is a brain scientist. Estrogen, particularly in its relationship to the health of the brain, is her obsession. At present it is mine too, but for more selfish reasons. We’re inside a darkened lab room in a research facility at the University of Southern California, where Brinton works. We are both in our 50s. I use estrogen, by means of a small oval patch that adheres to my skin, because of something that began happening to me nine years ago — to my brain, as a matter of fact. Brinton uses estrogen and spends her work hours experimenting with it because of her own brain and also that of a woman whose name, Brinton will say, was Dr. A. She’s dead now, this Dr. A. But during the closing years of her life she had psychologists. “We’d spend hours, me listening to her stories, and I’d walk out of the room,” Brinton told me. “Thirty seconds later, I’d walk back in. I’d say, ‘Dr. A., do you remember me?’ And she was so lovely. She’d say: ‘I’m so sorry. Should I?’ ”
The problem with the estrogen question in the year 2010 is that you set out one day to ask it in what sounds like a straightforward way — Yes or no? Do I or do I not go on sticking these patches on my back? Is hormone replacement as dangerous in the long term as people say it is? — and before long, warring medical articles are piling up, researchers are raising their voices and gesticulating excitedly and eventually you’re in Los Angeles staring at a fluorescent rodent brain in the dark. “You want a statistic?” Brinton asked softly. Something about the shooting mitochondria has made us reverent. “Sixty-eight percent of all victims of Alzheimer’s are women. Is it just because they live longer? Let’s say it is, for purposes of discussion. Let’s say it’s just because these ladies get old. Do we just say, ‘Who cares?’ and move them into a nursing home? Or alternatively, maybe they are telling us something.”
With their brains, she means. Their sputtering, fading Alzheimer’s brains, which a few decades earlier were maybe healthy brains that might have been protected from eventual damage if those women had taken estrogen, and taken it before they were long past their menopause, while their own neural matter still looked as vigorous as those rat cells on the wall. This proposition, that estrogen’s effects on our minds and our bodies may depend heavily upon when we first start taking it, is a controversial and very big idea. It has a working nickname: “the timing hypothesis.” Alzheimer’s is only one part of it. Because the timing hypothesis adds another layer of complication to the current conventional wisdom on hormone replacement, it has implications for heart disease, bone disease and the way all of us women now under 60 or so — the whole junior half of the baby boomers, that is, and all our younger sisters — could end up re-examining, again, everything the last decade was supposed to have taught us about the wisdom of taking hormones.
I first met Brinton at a scientific symposium at Stanford University in January that was entirely devoted to the timing hypothesis. The meeting was called Window of Opportunity of Estrogen Therapy for Neuroprotection, and it drew research scientists and physicians from all over the country. When I asked to listen in, the organizers hesitated; these are colleagues around a conference table, they pointed out. They’re probing, interrogating, poking holes in one another’s work in progress.
But I was finally permitted to take a chair in a corner, and as the day went on, I became aware of my patch, in a distracted, hallucinatory sort of way, as if I had started fixating on a smallish scar. One after another, their notes and empty coffee cups piling up around them, heart experts and brain experts and mood experts got up to talk about estrogen — experiments, clashing data, suppositions, mysteries. There are new hormone trials under way that are aimed at the 40-year-old to 60-year-old cohort, with first results due in 2012 and 2013. There are depression studies involving estrogen. There are dementia studies involving estrogen. There are menopausal lab monkeys taking estrogen, ovariectomized lab mice taking estrogen and young volunteers undergoing pharmaceutically induced menopause so researchers at the National Institutes of Health can study exactly what happens when the women’s estrogen and progesterone are then cranked back up. I typed notes into my laptop for hours, imagining the patch easing its molecules into the skin of my back, and the whole time I was typing, working hard to follow the large estrogen-replacement thoughts of the scientists around the table, I had one small but persistent estrogen-replacement thought of my own: If I make the wrong decision about this, I am so screwed.
I started taking estrogen because I was under the impression that I was going crazy, which turns out to be not as unusual a reaction to midlife hormonal upheaval as I thought. This was in 2001. The year is significant, because the prevailing belief about hormone replacement in 2001 was still, as it had been for a quarter century, the distillation of extensive medical and pharmaceutical-company instruction: that once women start losing estrogen, taking replacement hormones protects against heart disease, cures hot flashes, keeps the bones strong, has happy effects on the skin and sex life and carries a breast-cancer risk that’s worth considering but not worrying about too much, absent some personal history of breast cancer or a history of breast cancer in the immediate family.
At first, as I was trying to locate a psychiatrist who would take me on, I wasn’t aware I had reason to pay attention to advice about hormones at all. That year I turned 47, a normal age for beginning the drawn-out hormonal-confusion period called perimenopause, but I had none of the familiar signs. Menopausal holdouts run in the family; one of my grandmothers was nearly 60 by the time hers finally kicked in. My only problem was a new tendency to wake up some mornings with a great dark weight shoving my shoulders toward the floor and causing me to weep inside my car and basically haul myself around as if it were the world’s biggest effort to stand up straight and carry on a conversation. Except for its having shown up so arbitrarily and then coming and going in waves, there was nothing interesting about my version of what my husband and I came to think of as the Pit; anybody who has been through a depression knows what a stretch of semidisabling despair feels like, and for my part I had a very nice life, a terrific family and a personal interior chorus of quarreling voices demanding to know why I didn’t pull up my socks and carry on, which in fact was the first question I planned to ask a psychiatrist.
But I went to my gynecologist first, so she could check my blood pressure or whatever seemed the prepsychiatrist thing to do. How often would you say you feel this way, she asked; and I said I didn’t know, maybe every few weeks; and she told me to start keeping records. Note each day, she said. Check for patterns.
She was right. There was a pattern. I was falling into the Pit on schedule, around 11 days before each menstrual period, or M.P., which is one of many abbreviations I was to learn in my efforts to keep track of the ferocious hormones debate that started up in North America in 2002, one year after I stuck on the first estrogen patch that my gynecologist prescribed. The study at the center of the ruckus was called the Women’s Health Initiative, or W.H.I. It was a federally financed examination of adult women’s health, extraordinary in scale and ambition, that started up in the early 1990s; one of its drug trials enrolled more than 16,000 women for a multiyear comparison of hormone pills versus placebos. On July 9, 2002, W.H.I. investigators announced that they had ended the trial three years early, because they were persuaded that it was dangerous to the hormone-taking participants to let them continue.
The women on hormones were having more heart trouble than their placebo-taking counterparts, the investigators said, not less. Their risk for stroke went up. Their risk for blood clots went up. Their risk for breast cancer increased by 24 percent. The W.H.I. bulletins dominated medical news all summer and long into the fall, and so alarming were their broad-scale warnings that millions of women, myself included, gave up hormone replacement and resolved to forge ahead without it.
The patches my gynecologist prescribed worked, by the way. I didn’t understand how, beyond the evident quieting of some vicious recurring hormonal hiccup, and neither did the gynecologist. But she had other women who came in sounding like me and then felt better on estrogen, and I would guess many of them, too, decided after the W.H.I. news that they could surely find other ways to manage their “mood swings,” to use the wondrously bland phrasing of the medical texts. (I’m sorry, but only someone who has never experienced one could describe a day of “I would stab everyone I know with a fork if only I could stop weeping long enough to get out of this car” as a “mood swing.”) We muddled along patchless, my mood swings and my patient family and I, until there came a time in 2006 when in the midst of some work stress, intense but not unfamiliar, I found myself in a particularly bad Pit episode and this time unable to pull out.
It was profoundly scary. In retrospect, I managed a surprising level of public discretion about what was going on; competence at the cover act is a skill commonly acquired by midlife women, I think, especially those with children and work lives. If the years have taught us nothing else, they have taught us how to do a half dozen things at once, at least a couple of them decently well. Like other women I have met recently with stories like this one, I relied for a few months on locked office doors, emergency midday face-washings and frequent visits to an increasingly concerned talk therapist. But one afternoon I got off my bicycle in the middle of a ride with my husband, because I had been crying so hard that I couldn’t see the lane lines, and I sat down on the sidewalk and told him how much I had come to hate knowing that family obligations meant I wasn’t allowed to end my life. The urgent-care people at my health clinic arranged a psychiatric consult fast, and after listening and nodding and grabbing scratch paper to draw me an explanatory graph with overlapping lines that peaked and plunged, the psychiatrist wrote me two prescriptions. One was for an antidepressant.
The other — I recognized the name as soon as she wrote it down — was for Climara, my old estrogen patch.
By this time we were four years past the 2002 W.H.I. hormone news. So I knew a few more things. I knew there had been a surge of industrious scrambling among former hormone-taking women, some of whom had tried multiple alternatives or going cold turkey and then changed their minds and re-upped on estrogen, deciding that life without it was so unpleasant that they no longer cared what the statistical prognoses said. I knew the prevailing medical sentiment had shifted slightly since the bombshell of 2002; certain articles and books still urged women to shun hormone replacement at all costs, but the more typical revised counsel was, essentially, proceed with great caution. If some menopausal malady is genuinely making you miserable, the new conventional wisdom advised, and no alternative remedy is working for you, then go ahead and take hormones — but keep the dose low and stop them as soon as possible.
I would like to be able to tell you that I weighed these matters thoughtfully, comparing my risks and benefits and bearing in mind the daunting influence of a drug industry that stands to profit handsomely from the medicalizing of normal female aging. But that would be nonsense, of course. I was too crazy. I went straight to the pharmacy and took everything they gave me.
You don’t read the fine print on package labels when you’re being ushered through a psychiatric crisis, but after a while, I did. By last winter I was nearing the cumulative five-year mark as an estrogen user, and although “low dose, stop soon” is often an advisory without specifics attached, five years seemed to turn up here and there as an informal outer-limit guideline. And because it had worked again, because the estrogen so clearly helped repair something that was breaking (there’s no way for me to separate the effects of estrogen from the effects of the antidepressant, except that on the few occasions when I’ve been haphazard about replacing the estrogen patches on time, I’ve experienced prompt and unmistakable intimations of oncoming Pit), I now had some rational faculties with which to go looking for explanations that might help me decide what to do. This was when I first began learning that in the controversy over hormone replacement, the fine print matters a very great deal.
First of all, the kind of estrogen in my patches — there are different forms of estrogenic molecules — is called estradiol. It’s not the estrogen used in the W.H.I. study. Pharmaceutical estradiol like mine comes from plants whose molecules have been tweaked in labs until they are atom for atom identical to human estradiol, the most prominent of the estrogens premenopausal women produce naturally on their own. The W.H.I. estrogen, by contrast, was a concentrated soup of a pill that is manufactured from the urine of pregnant mares. The drug company Wyeth (now owned by Pfizer) sells it in two patented products, the pills Premarin and Prempro, and it’s commonly referred to as “conjugated equine estrogens.”
There was more in the fine print. Two years ago, after warning me that women who haven’t had a hysterectomy run a higher risk of uterine cancer when they take only estrogen as hormone replacement, a new doctor added in progesterone, which has been shown to protect the uterus. The progesterone he prescribed for me, like the estradiol, is a molecular replica of the progesterone women make naturally. It’s different from the progesteronelike synthetic hormone that was used for the W.H.I. study that ended in 2002. That medication was a formulation whose multisyllabic chemical name shortens to MPA and which has a problematic back story of its own: MPA takes care of the uterine-cancer risk, but there’s reason to suspect it may be a factor in promoting breast cancer. And it’s ingested as a pill, which means that like equine estrogens (and unlike, for example, my patch), MPA metabolizes through the liver, possibly creating additional complications en route, before going about its business.
The biggest difference between me and the W.H.I. women, though, has to do with age and timing. I started on the patches while my own estrogen, pernicious though its spikes and plummets may have been, was still floating around at more or less full strength. The average age of the W.H.I. women was just over 63, though the study accepted women as young as 50. More significant, though, most of them were many years past their final menstrual period, which is the technical definition of menopause, when they began their trial hormones. The bulk of the group was at least 10 years past; factoring in the oldest women, the average number of years between the volunteers’ menopause and their start on the trial medications was 13.4.
Because women generally make decisions about hormones while they are in the throes of perimenopause — that term is now used to extend through the year following the final M.P. — you may find this as perplexing as I did. Why would the largest drug trial in the history of women’s health select, for most of its participants, women already long past the critical phase? I heard one undiplomatic critic sum up the W.H.I. as “the wrong drugs, tested on the wrong population,” and those two factors, the drugs and the population, are actually directly linked. Equine estrogens and MPA were the only forms of hormones used in the W.H.I. trials. Among other reasons, that’s because drug trials are expensive; this one was huge, and Wyeth was going to provide without cost an average of eight years’ worth of its equine estrogens and MPA to 40 clinical centers.
And millions of women were using those very hormones already, partly because aggressive Wyeth marketing had for three decades insisted that hormone replacement was the ticket to a vigorous and sexually satisfactory postmenopausal life. To a certain extent, evidence backed up that claim; wide-scale though less rigorous earlier studies appeared to demonstrate hormone replacement’s benefits so clearly that many physicians were suggesting it almost automatically to midlife women, whether or not they had perimenopausal complaints. Hormones raised the breast-cancer risk in those earlier studies, but nearly every other health factor showed improvement when women who took hormones were compared with those who didn’t. Hot flashes disappeared, osteoporosis was milder, women reported feeling better and women who took hormones showed a markedly lower rate of heart disease than women who did not.
Because heart disease ultimately kills many more women than all cancers combined, some doctors had also taken to urging older women, even those past menopause, to start hormones for cardiac-health purposes. The W.H.I. trials were supposed to provide conclusive evidence, finally, as to whether all this wide-scale prescribing was truly a sound idea. But cardiovascular disease tends to make its bids for attention — its “events,” as clinicians say, like heart attack and death — when we’re quite a bit past 51, the average age at which American women hit menopause. The only way the W.H.I. was going to tally up a scientifically useful number of cardiac events was to enroll plenty of women already old enough to reach that danger stage before the study’s time ran out. So that’s what they did, and once the final data was reparsed many times, it was clear that the trial had shown physicians something highly important about the perils of starting older postmenopausal women (that’s qualifier No. 1) on pills (No. 2) containing equine estrogens (No. 3) plus MPA (No. 4).
Those four qualifiers make the chief message of the W.H.I. — that taking hormones, in the long run, is more likely to hurt you than help — far more specific than the one most women heard. For those of us not yet on the far side of menopause, or who don’t match the other qualifiers (as I write this, for example, I’m zero for four), a daunting proportion of what we thought we learned about hormone replacement over the last eight years remains unsettled, more confusing than ever and conceivably — we don’t know yet — wrong. “I mean, if you’re a 70-year-old,” says S. Mitchell Harman, a Phoenix-based endocrinologist and coordinator of one of the national trials currently examining hormones’ effects on younger women, “and your question is, Should I start taking estrogen? the W.H.I. answered that for you beautifully. No. Unfortunately, it wasn’t designed to answer that question for a 50-year-old. So now we’re trying to fill in the blanks.”
One afternoon last month, I reported to the Northern California site for an N.I.H.-financed cognitive trial that is part of the Kronos Early Estrogen Prevention Study that Harman is leading. Keeps, as it’s called, has enrolled women at nine such sites around the country; this one was inside a medical building at the University of California, San Francisco, and the cognition test I asked to try proved to be a low-tech experience: a table with chairs, pens and pencils and a gentle-voiced psychologist asking me to do things with my brain. Number sequences repeated backward, lists of random objects to recall, designs to remember and copy — I promised not to describe specifics, because making details public could compromise the trial results. But imagine a stranger holding up a stopwatch and giving you 30 seconds to name every dessert item you can think of. The brain charges off into a comical panic grope, and it’s like a cross between a back-seat car game and the SATs.
The only grading marker, though, is self compared to self. If I were a Keeps participant, I would be on a four-year regimen of some mystery medication — either estrogen, in one of two forms (estradiol patches or equine-estrogen pills, to see whether differences emerge between the two), or placebo patches or placebo pills. Then in another year, I would retake the cognition test, which lasted about an hour and a half, so researchers could track any change. Brain function is a major element of the Keeps agenda; the other is heart health, so the test administrators would conduct annual ultrasounds of my carotid artery, to check for the thickening that signals heart disease. That’s how they are trying to circumvent the doesn’t-manifest-until-you’re-older problem, by measuring for known warning markers rather than waiting for the actual big events. They would check my blood and cholesterol for signs of other cardiovascular trouble.
With about 730 participants, Keeps is relatively small; hormone research has been tough to finance in the post-W.H.I. years, and every scientist and physician I’ve spoken to said there will never again be another hormone trial as costly and ambitious as the W.H.I. A second study, based in Los Angeles, called the Early Versus Late Intervention Trial With Estradiol, is following more than 600 women — comparing a group that has been post-menopausal for an average of 15 years and that is on estradiol or on a placebo with a second, younger group that is an average of three years post-menopausal. “This is the age when we should really study estrogen,” says Sanjay Asthana, a University of Wisconsin medical professor who is a designer of the cognition component of Keeps. “People like me are really waiting to see what this data looks like. Either way. We need to know.”
Asthana is a geriatrician, with a specialty in Alzheimer’s and other forms of age-related memory loss. That makes him a member of what I came to think of, in my travels among estrogen researchers this winter, as the brain contingent. Their working material includes neuroimaging; magnified slices of rodent brains; and live cells that carry on in petri dishes, shooting mitochondria around or struggling under the burden of disease. All these things allow the brain contingent to see, sometimes literally, estrogen in action. It’s an amazing process. When cells are healthy, estrogenic molecules slide right in, searching for special receptors that are shaped precisely for the estrogens: the receptors are tiny locks, waiting for the right molecular keys to turn them on. Then, once they are activated by the key-turning process, the work estrogen receptors do is richly complex, if only partly understood. They prod genes into action; they raise good cholesterol; they affect the neurotransmitter chemicals associated with mood and stress, like serotonin and dopamine.
And the brain, scientists have learned in recent decades, is loaded with these receptors. Knowing this makes it easier to understand how perimenopause could start inside aging ovaries and set off such a wild cascade of effects. If you’re a typical woman moving through your 40s or 50s, your lifetime egg supply is running out; as that happens, the intricate, multihormone reproductive-signaling loop grows confounded, its triggers altered by the biology of change. The brain and ovaries, the primary stops along this loop, start misreading each other’s demands for action. This can make estrogen production crank up frantically, crash and then crank up again. Something also goes awry with most women’s thermoregulatory systems, producing hot flashes in around three-quarters of us — nobody yet knows why, exactly, nor why certain women go on flashing for many years while some escape the whole must-remove-outer-garments-now
Not all women, Manson notes, experience disruptions as robust as this unidentified patient’s. But consider the mess of internal rearrangement we’re looking at: the body’s overall estrogen production is waning as the ovaries start atrophying into full retirement; and here simultaneously, at least for some of us, is this great Upheaval of During. The combination of the two can be — how could it not, I thought, the first time I studied the three graphs — a hellacious strain on the brain. Tracing the exact mechanics is still a work in progress, but they surely include some disruption of signaling to the neurotransmitters that make us remember things, experience emotions and generally choreograph the whole thinking operation of the human self.
“There are all these fundamental cognitive functions that many perimenopausal women complain about, and one of those fundamentals is attention,” Roberta Brinton, the U.S.C. scientist, told me. “When you can’t hold your attention to a thought. Where you’re in constant start mode, and you never reach the finish mode. That is devastating.”
This was Brinton, as it happens, describing herself. It’s why she first went on estrogen (estradiol, accompanied by natural progesterone) when her own perimenopause kicked in a few years ago. We were sitting in a campus garage in her Prius one day, and I asked her what made her so sure her own midlife difficulties — she had the hot flashes, which were obvious, but also the sleep disruption and the infuriating distractibility — were the product of hormonal events, not some womanly existential crisis. We get a lot of that, societally. It’s meant to be empathetic. Your role in life is changing, Mrs. Brain Seized by Aliens! Your children are growing up, you’re buying expensive wrinkle cream, ice cream makes you gain weight now, of course you’re distraught! “Because with estrogen — ” Brinton looked at me sharply, and then smiled — “I don’t have attention-deficit disorder.”
We walked back up to her laboratories, which are spread along a many-roomed warren full of cell incubators, centrifuges and computers. Brinton has thick black hair and a demeanor of lively, good-humored authority; it’s easy to envision her as the passionate science professor in crowded lecture halls. But in her labs the work is all rats and mice, many of them surgically or genetically altered to serve as surrogates for adult humans in various stages of maturation or disease. Removing the ovaries from female rats, for example, sends them into low-estrogen mode. Mice can be ordered bred with Alzheimer’s. The plaque that clogs the brains of Alzheimer’s sufferers, a noxious memory-disrupting substance called beta amyloid, is available as a chemical distillate, which means Brinton’s team can experiment with that too — beta amyloid dropped into the brain cells of healthy low-estrogen rodents; or estrogen dropped into cells already damaged by beta amyloid.
That’s why Brinton says that the timing hypothesis — the proposition that estrogen could bring great benefit to a woman who starts it in her 50s while having the reverse effect on a woman 10 years older — makes sense even though it is still experimental. She and other scientists know there are ways estrogen improves and protects the brain when it is added to healthy tissue. It makes new cells grow. It increases what’s called “plasticity,” the brain’s ability to change and respond to stimulation. It builds up the density and number of dendritic spines, the barbs that stick out along the long tails of brain cells, like thorns on a blackberry stem, and hook up with other neurons to transmit information back and forth. (The thinning of those spines is a classic sign of Alzheimer’s.)
But when estrogen hits cells that are already sick — because they’re dying off as part of the natural aging process or because they’ve been damaged by beta amyloid — something else seems to happen. Dropped in as a new agent, like the wrong kind of chemical solvent sloshed onto rusting metal, estrogen doesn’t strengthen or repair. It appears useless. Sometimes it sets off discernible harm. You may recall additional W.H.I. news a few years ago about hormones increasing the risk for aging-related dementia; those stories emerged from a subgroup of W.H.I. participants who were all at least 65 when they started the hormones. There are arguments about that data, like nearly everything else connected to the W.H.I., but the age factor alone reinforces what Brinton and other timing-hypothesis researchers observe in the labs when they give estrogen to ailing cells. “It’s like the estrogen is egging on the negative now, rather than the positive,” she said. “We know that if you give neurons estrogen, and then expose them to beta amyloid, many more will survive. But when we expose them to amyloid and then give them estrogen — now you don’t have survival of the neurons. In some instances, you can actually exacerbate their death.”
The heart contingent exploring the timing hypothesis is reasoning the same way. Monkeys get both cardiovascular disease and their own version of menopause; there is a primate team at Wake Forest University in North Carolina that has found estrogen to be a strong protectant for females against future heart disease — but only when it’s given at monkey perimenopause. Give estrogen the equivalent of six human years later, says Tom Clarkson, the pathology professor who has been leading this work for decades, and there is no protective effect at all.
Clarkson, who is 78, told me that if he were 30 years younger and a woman, with hot flashes or sleep trouble or sudden crashes of mood, he would have no hesitation about taking hormones. “I absolutely believe in the timing hypothesis,” he said. Then, being a scientist, he corrected himself. “I would have to say my level of certainty is 95 percent or greater,” he said. “I live a life of believing in the experimental evidence.”
So noted, I replied. And what if the symptoms were annoying but bearable or there were no symptoms at all? I’ve asked the same questions to every researcher I talked to this spring, and nearly all of them reply the same way: if they were deciding for themselves personally, they would tip the risk-benefit scale strongly in favor of hormones as a remedy for immediate ailments of perimenopause. But estrogen solely as a protectant for the heart and brain, to be taken for many years, absent any immediate serious complaints? There was a pause, and I heard Clarkson sigh. “We just don’t know about that yet,” he said.
The personal calculus of risk is an exhausting exercise in the modern era, what with litigation-jumpy physicians, the researchers’ candid “We just don’t know” and the bottomless learn-it-yourself maw of the Internet. Of all the conversations I had this winter, as I weighed and reweighed the stopping of the patch, the one that most resonates took place on a snowy morning in Washington, in the office of a nursery-school director named Julia Berry. Berry lives not far from the headquarters of the National Institutes of Health in Bethesda, Md., which is why last September she pulled from her mailbox a card the N.I.H. has been mailing to local women within a certain age range. “If you struggle with irritability, anxiety, sadness or loss of enjoyment at the time of the menopausal transition,” the card reads, “please call us and help yourself while helping others.”
The N.I.H., it turns out, has been quietly conducting mood and hormone studies for more than two decades under the direction of a psychiatrist named Peter Schmidt and his predecessor, David Rubinow, who is now chairman of the psychiatry department at the University of North Carolina. The research was first set into motion by Rubinow’s postgraduate interest in premenstrual syndrome; the idea of giving younger women drugs to lower and flatten temporarily their estrogen and progesterone levels, essentially inducing menopause, was initially conceived to determine the role of hormones in PMS — to see whether these young women got relief when their hormones stopped the cresting and dropping of the normal menstrual cycle. (It often worked as a short-term treatment and yes, the young women often got hot flashes.) In recent years, the induced-menopause experiments have continued, among many other studies, as part of an effort to try to understand the chemistry of women like Julia Berry and me — women for whom perimenopause turns into what Berry described to me as “psychological misery, not myself and absent from the world.”
Berry is 55, ponytailed and roundish and pretty. She was divorced a long time ago, raised three good kids mostly on her own and has a firm handshake and a job she loves. Her troubles started in her late 40s, in the standard way, with hot flashes and jerking awake at 3 a.m. and then escalated into something much fiercer. Like me, at the worst of it, she occasionally found herself in traffic, wishing silently for an oncoming truck that might exit her swiftly from this life without qualifying as a suicide. A physician prescribed antidepressants. They helped, with both the anguish and the flashes, but not enough. “I am one of the most steady, even-keeled, hard to ruffle, really unflappable . . . truly,” Berry told me. “I am. I, generally speaking, can be completely relied upon to do the sensible right thing almost all the time. Which is one of the reasons this period in my life has been so weird.”
She called the N.I.H. number at once. She was quickly evaluated, enrolled in a double-blind study of the effects of estrogen on perimenopausal depression and sent home with a paper bag containing a mystery patch. When I asked Berry to describe the sensation of the next few weeks, she looked up at the ceiling for a second to think. “Kind of like having been in a smoky room, waving your arms and now seeing that the exhaust fan is taking a little at a time,” she said. “My mood lifted. First time in three years I wasn’t waking up at 3 in the morning. That’s when I knew I wasn’t on the placebo. It was very clear to me that there was something fundamentally wrong with my chemical systems, and that whatever was in this patch was setting things right, so that I could function like a regular human being — the human being I was familiar with.”
What medicine doesn’t know about the chemistry of mood, including clinical depression, dwarfs what medicine doesn’t know about hormones. It would be handy for science if Berry and I could have made our heads available for dissection at certain points in recent years; as it is, we’re able to answer as many elaborations on “I feel bad” or “I feel good” as researchers might wish to throw at us, but they still have no way of pinning down where we belong on the scale of menopausal distress, or what exactly we’re doing there. We could be extra-high-volume versions of the women who are having an ordinary rough time of it, like Roberta Brinton — the women who hot-flash and can’t sleep and cast about for vocabulary with which to describe feeling, as Brinton puts it, “just off.” Or we could belong to some subcategory of anomalies, women with a wired-in susceptibility to depression — gene pools, childhoods, whatever — that was fired up by abrupt hormonal change.
Some psychological surveys will tell you there’s no evidence for a surge of clinical depression at menopause. I believe that, given how many other phases of life can unhinge us, but I also believe — no, actually, I know — that there is a difficult thing that happens to some women in the perimenopausally affected brain. Hostile as I am to generalizations involving women rendered fragile by biology, here I am, and here, too, is Berry, both of us pulled out of something terrible by a pharmaceutical infusion of estrogen. Two physicians who specialize in hormones and mood, Louann Brizendine, a neuropsychiatrist at the University of California, San Francisco, and Claudio Soares, a Canadian research psychiatrist who works at McMaster University in Ontario, told me that women who seek them out tell variations of the same story Berry and I took to our doctors: I know that something is wrong with me because I also know, somewhere in the noncrazy part of myself, that there is such pleasure to be offered by the circumstances of my grown-up life.
“These women thought they were losing their minds,” Brizendine told me, describing the 40-to-60-year-old patients she began seeing when she opened the Women’s Mood and Hormone Clinic at the university in 1994. “In 1994 we didn’t have words for it,” she said. “Now we do. It’s called perimenopausal depression.”
Brizendine and Soares, like Schmidt and Rubinow, have found that various combinations work with varying degrees of effectiveness for many of us — hormones with an antidepressant, hormones without an antidepressant, sometimes antidepressants on their own. The alternatives-to-hormones recommendations are mostly fine things in their own right, varying from certainly useful to harmless: exercise regularly, keep the weight down, easy on the caffeine, calm yourself with deep breathing or yoga, try black cohosh. (You could start a bar brawl over the efficacy of black cohosh, but the general consensus seems to be: if it works for you, go for it.) But the troubles set off by ricocheting hormones are reliably fixed by making the hormones stop ricocheting. And the laborious weighing of hormones’ benefits versus hormones’ harms — maybe not at the crisis moment, for those of us at our most distraught, but later, one or two or five years down the road — is something still undertaken by millions of women along the full breadth of the perimenopausal spectrum.
How in the world to do it wisely enough so the calculation is as right for each of us as it can possibly be? JoAnn Manson’s book contains the most careful checklist I’ve seen yet; by the time you answer all the personal-history questions the book asks you to consider, you’ve read 82 pages. Breast cancer is a factor, to be sure, but so are colorectal cancer, ovarian cancer, stroke, hip fracture and diabetes. If the timing hypothesis proves right and estrogen really does protect our brains and our hearts as long as we start it early enough, the calculation only grows that much more important and complex. There are moving pieces involved in working out every one of these risks in relation to everything else, and anyone who thinks there’s a bumper-sticker answer to the hormones question — don’t take them, you’re sure to be better off — is, like me that day in the psych unit, neither listening to scientific argument nor reading the fine print.
Here’s one example from the many to which researchers have pointed me this winter. Remember MPA? The synthetic progesteronelike substance used along with equine estrogens in the W.H.I.? There was a second W.H.I. hormones-versus-placebo trial, of nearly 11,000 women, that was also started in the early 1990s, just like the one that was halted in 2002. All the women enrolled in this second study had undergone hysterectomies, which meant they had zero risk for uterine cancer. So the women on medications in this trial were taking only equine estrogens — no MPA, which you’ll recall is given to protect the uterus. Their study was stopped in 2004, also before its planned end date, because the estrogen-taking women were showing a higher risk of stroke than the women on the placebo. But their breast-cancer rate was lower. The hormone-taking women with hysterectomies in that second study, who used estrogen without MPA, showed a 23 percent lower risk of invasive breast cancer than their counterparts who were taking no hormones at all.
Nobody’s persuaded that this means MPA promotes breast cancer while estrogen does not. It’s clear that estrogen acts aggressively on certain breast malignancies and that any woman who has had breast cancer or has a history of it in her immediate family should stay off estrogen. This is one of the principal reasons such intense work is under way right now, in labs like Roberta Brinton’s, to develop estrogenic variants — molecular substances designed to latch only to certain receptors (in the brain, say, where the activated receptors can do their good works) while ignoring receptors in the breast and uterus. And there are plenty of confounding factors, as scientists say, with regard to the women in the no-MPA trial. They all had undergone hysterectomies, for one thing; maybe whatever caused them to require uterine removal in the first place affected their reactions to the estrogen.
Or it could have been a fluke. But the MPA wrinkle adds suspicion and urgency to the timing-hypothesis questions about what really goes on when women of our demographic use hormones, and Julia Berry and I spent a long time talking about this, the adding and subtracting, the guessing and weighing, the balancing of what we think we know about ourselves against what we cannot possibly foresee. We will both, for the present, continue wearing estrogen patches. Berry turned out to be right, of course; she wasn’t on the placebo, which the N.I.H. doctors told her when she finished the study. And as she hurried to fill her own patch prescription, she found her gratitude mixed with more than a little frustration. “Why did my primary-care physician give me an antidepressant when I could have had something simple, like estrogen?” she asked. “Why don’t they know?”
We talked about breast cancer, because that is the nightmare illness in nearly all our calculations, for most of us the visual closest to hand. Three of my best friends have endured the full breast-cancer horror show and by now have retired their wigs. All have survived. None had been on hormone replacement. This is information that batters me steadily but not helpfully, like my ex-smoker paternal aunt’s fatal lung cancer and the fact that I’m a lifetime nonsmoker and regular exerciser with extremely good cholesterol levels. How do my lowered risks from one column balance against my question marks over in another column? What to do?
“I’d rather monitor something I know can go wrong than go on living in the state I was in,” Berry said. “I could have my breasts removed. I like them. But they’re not my life.”
We’ve spent a fair bit of time by now, Julia Berry and I, shaking these uncertainties out and squinting at them. Do we wear these patches forever? We don’t know. What happens when we do take them off, if we do? We don’t know. Have we done nothing except delay a biological process, complete with hot flashes and another round of truck-crash fantasies, that at some point we’ll have to bully our way through? We don’t know, nor does any researcher I talked to this spring.
And there’s this: Should luck and longevity cooperate, we are going to grow old. We’re already old, by the standards of our children and our ancestors, but the generation to which we belong expects to live a rich messy life full of extremely loud rock music for another 30 years after menopause. Every midlife woman I know keeps redrawing for herself the defensible lines of intervention in the “natural” sequence of human aging. Obsessive multiple plastic surgeries are silly and desperate. Muscles kept in good working order are not. Where on that spectrum is a hormones-saturated pharmaceutical patch? What if the timing hypothesis is even partly right? Suppose all we learn about replacement estrogen, in the end, is that if it’s started early enough it might protect the heart and the brain, and that its chemistry makes some of us feel more the way we did at 40 than the way our mothers did at 65? Not an elixir of youth. More like . . . reading glasses. Or calcium supplements, or painkillers that stop the knee from hurting but carry risk warnings of their own. It has occurred to me that the better analogy might be a 13-year-old trying to ward off puberty by binding her breasts, but most of the time I don’t think so, and if I do try stopping the patches, I know this to a certainty: I will keep a few extras in reserve, just in case.
Cynthia Gorney is a contributing writer to the magazine. She teaches at the Graduate School of Journalism at the University of California, Berkeley.
When Anxiety, Insomnia Aren't Just in Your Head
For Some Women in Their 40s, They're Among the Symptoms That Signal a Shift in Hormone Levels Before Menopause
By Melinda Beck : WSJ : October 12, 2010
One of the first things to go is often a good night's sleep.
That alone would make someone edgy, irritable and exhausted. But then come heart palpitations, difficulty recalling familiar words, loss of sex drive, mood swings and anxiety.
Women who encounter these symptoms in their 30s and 40s are often prescribed sleeping pills, tranquilizers, anti-depressants or anti-anxiety medications.
Yet all of these symptoms and more—migraines, joint and muscle pain, dry skin, thinning hair, weight gain and digestive problems—can be due to fluctuating levels of the hormones estrogen and progesterone that start as many as 10 years before menopause.
Easing the Transition
Women experiencing symptoms of perimenopause should undergo a comprehensive exam to measure blood pressure, cholesterol and bone density, as well as thyroid and other hormone levels. Doctors can prescribe treatments, if necessary, to alleviate these symptoms.
This life phase, called perimenopause or menopausal transition, begins when a woman's monthly periods first become erratic, and it's increasingly recognized as the time when symptoms can be most severe. There's also a growing awareness that those symptoms go well beyond the hot flashes, vaginal dryness and bone loss typically associated with menopause, to include a wide range of emotional, cognitive and physiological functions affected by estrogen.
Not every woman experiences these symptoms, and not all doctors think to attribute insomnia, mood changes and memory problems to hormonal shifts. But some physicians are treating women who are bothered by perimenopause symptoms with birth-control pills to even out erratic monthly cycles. Exercise, diet and lifestyle changes can also provide limited relief.
During perimenopause, estrogen receptors throughout the brain are affected by changing levels of the hormone—including regions that regulate sleep, temperature control, blood pressure and heart rate. "Women will wake up wide awake with their heart racing, then settle down and about 90 minutes later, it will happen again. That's a characteristic brain symptoms of declining estradiol," the form of estrogen, made in the ovaries that declines in menopause, says Elizabeth Lee Vliet, a women's health physician in Dallas and Tucson, Ariz.
Estrogen also affects the action of serotonin and norepinephrine, the neurotransmitters that are important in regulating mood, which help explain why numerous studies have found that rates of low-grade depression are particularly high in women in the years leading up to menopause.
"Many of these women are told, 'You feel bad because your husband is having an affair, or your youngest is in college, or you didn't get that promotion. Take this Prozac.' When what is really going on was fluctuating levels of estrogen," says Steven Goldstein, a professor of obstetrics and gynecology at New York University's Langone Medical Center and incoming president of the North American Menopause Society (NAMS).
A few small studies have shown that estrogen is as effective as anti-depressants at treating perimenopausal depression, and that some women benefit from a combination of the two.
Studies of cognitive function during perimenopause have been more mixed. "Women don't suddenly start functioning less well as menopause approaches," says Margery Gass, executive director of NAMS.
But Gayatri Devi, a neurologist and psychiatrist in New York City, says that tests of cognitive function often aren't subtle enough to measure the kind of word-recall problems and fuzzy thinking that some women experience before and after menopause. She frequently finds that estrogen replacement or birth-control pills ease some memory problems.
Researchers at University of California-Los Angeles, analyzing data from the multi-center Study of Women's Health Across the Nation, found that two-thirds of 2,300 women aged 45 to 57 experienced some memory problems during their menopausal transition time, and that those who began hormone-replacement therapy before their last period had more improvement than those who waited.
Birth-control pills or patches can alleviate perimenopausal symptoms by putting a woman's natural ovarian function at rest and providing a steady level of hormones that keep brain function on an even keel. They also prevent pregnancy, which can be more problematic with other methods when periods have become unpredictable.
The pill does have some downsides—including a slightly higher risk of breast cancer in some cases. Women over 35 who smoke or have tested positive for a gene mutation that predisposes them to breast cancer should not take oral contraceptives. But birth-control pills also lower the risk of uterine and ovarian cancer.
Dr. Goldstein notes that today's pills contain only a half to a quarter of the amount of those in decades past, and because they suppress natural ovulation, women are exposed to lower amounts of estrogen while on a low-dose pill than they would if they were ovulating normally.
He often suggests that women who are encountering symptoms try going on birth-control pills for two months to see how they feel. "I've seen people who have significant improvement. I've seen women who have moderate improvement and women who have no improvement," on the pill, Dr. Goldstein says.
The proportion of hormones varies in pills. Dr. Vliet notes that if women taking a very-low estrogen pill still have headaches, depression, low libido or fatigue, they should ask about switching to a brand with more estrogen and less progestin, such as Femcon, Ovcon 35, Ortho-Cyclen or Yasmin.
A more complicated option is to give perimenopausal women estrogen and progesterone to augment the decline in what their ovaries are making—ideally using an estradial patch or gel and a natural progesterone.
Some doctors, like Dr. Vliet, believe in checking specific hormone levels with blood tests; others say there are too many variations in blood levels and go by symptoms instead.
For women who don't want to take hormones, a few lifestyle changes can help with perimenopausal symptoms. Exercise helps ward off weight gain and enhance serotonin and endorphins. Eating a balance of proteins and carbohydrates can keep weight and blood sugar on an even keel. Drinking alcohol in moderation can lower the risk of heart disease, but drinking more than one glass a day raises the risk of breast cancer and can interfere with sleep.
"I know women who drink a half a bottle of wine every night to deal with their perimenopause symptoms, but they are raising their risk of breast cancer far more than taking estrogen would," says Dr. Vliet.
As for herbal remedies such as black cohosh, wild-yam root and dong quai, the limited studies that have been done have not shown them to have any more benefit than placebos.
If a perimenopausal woman does use birth-control pills, how long should she stay on them? Dr. Goldstein suggests that women stop temporarily when they reach age 51 to see if their natural periods return. If so, he suggests that women return to the pill and try stopping again in six months.
Going off birth-control pills at menopause can bring on the very symptoms they were preventing earlier. In many women, those symptoms will subside. "In a way, you can compare it to puberty—a lot of people have irregular cycles then, and they are more moody, but it generally goes away in a few years," says Dr. Gass.
If bothersome symptoms persist, women can transition into hormone-replacement therapy, which uses a much lower dose of hormones than birth-control pills. The key is to start early, during or soon after menopause. While the Women's Health Initiative (WHI) study found that hormone therapy raised the risk of cardiovascular disease, stroke and breast cancer, the women studied were generally over 65 years old and 10 years past menopause. Subsequent analyses of the data showed that women who started on hormone therapy within five years of menopause had a protective effect instead.
Marketers of so-called bio-identical hormones made in compounding pharmacies often say their products alleviate many of the cognitive and mood symptoms of perimenopause and are safer than standard prescription forms. Yet many of the same "bio-identical" hormones are available in prescription forms that have been approved and inspected by the Food and Drug Administration, unlike combinations from compounding pharmacies. Indeed, another study presented at the NAMS annual conference in Chicago last week found that hormone products made by compounding pharmacies often contained lower doses of hormones than their labels suggested.
All women experience perimenopause differently. The best first step is to undergo a comprehensive exam from an ob/gyn or primary-care doctor to assess blood pressure, cholesterol, bone density and hormone levels including thyroid, all of which are affected by estrogen. Heavy bleeding in perimenopause can be a symptom of a serious condition, such as uterine cancer.
If doctors still dismiss symptoms as "all in your head," or simple aging, persist or find another doctor. "Trust your symptoms," says Dr. Devi, "and seek help accordingly."
For Some Women in Their 40s, They're Among the Symptoms That Signal a Shift in Hormone Levels Before Menopause
By Melinda Beck : WSJ : October 12, 2010
One of the first things to go is often a good night's sleep.
That alone would make someone edgy, irritable and exhausted. But then come heart palpitations, difficulty recalling familiar words, loss of sex drive, mood swings and anxiety.
Women who encounter these symptoms in their 30s and 40s are often prescribed sleeping pills, tranquilizers, anti-depressants or anti-anxiety medications.
Yet all of these symptoms and more—migraines, joint and muscle pain, dry skin, thinning hair, weight gain and digestive problems—can be due to fluctuating levels of the hormones estrogen and progesterone that start as many as 10 years before menopause.
Easing the Transition
Women experiencing symptoms of perimenopause should undergo a comprehensive exam to measure blood pressure, cholesterol and bone density, as well as thyroid and other hormone levels. Doctors can prescribe treatments, if necessary, to alleviate these symptoms.
This life phase, called perimenopause or menopausal transition, begins when a woman's monthly periods first become erratic, and it's increasingly recognized as the time when symptoms can be most severe. There's also a growing awareness that those symptoms go well beyond the hot flashes, vaginal dryness and bone loss typically associated with menopause, to include a wide range of emotional, cognitive and physiological functions affected by estrogen.
Not every woman experiences these symptoms, and not all doctors think to attribute insomnia, mood changes and memory problems to hormonal shifts. But some physicians are treating women who are bothered by perimenopause symptoms with birth-control pills to even out erratic monthly cycles. Exercise, diet and lifestyle changes can also provide limited relief.
During perimenopause, estrogen receptors throughout the brain are affected by changing levels of the hormone—including regions that regulate sleep, temperature control, blood pressure and heart rate. "Women will wake up wide awake with their heart racing, then settle down and about 90 minutes later, it will happen again. That's a characteristic brain symptoms of declining estradiol," the form of estrogen, made in the ovaries that declines in menopause, says Elizabeth Lee Vliet, a women's health physician in Dallas and Tucson, Ariz.
Estrogen also affects the action of serotonin and norepinephrine, the neurotransmitters that are important in regulating mood, which help explain why numerous studies have found that rates of low-grade depression are particularly high in women in the years leading up to menopause.
"Many of these women are told, 'You feel bad because your husband is having an affair, or your youngest is in college, or you didn't get that promotion. Take this Prozac.' When what is really going on was fluctuating levels of estrogen," says Steven Goldstein, a professor of obstetrics and gynecology at New York University's Langone Medical Center and incoming president of the North American Menopause Society (NAMS).
A few small studies have shown that estrogen is as effective as anti-depressants at treating perimenopausal depression, and that some women benefit from a combination of the two.
Studies of cognitive function during perimenopause have been more mixed. "Women don't suddenly start functioning less well as menopause approaches," says Margery Gass, executive director of NAMS.
But Gayatri Devi, a neurologist and psychiatrist in New York City, says that tests of cognitive function often aren't subtle enough to measure the kind of word-recall problems and fuzzy thinking that some women experience before and after menopause. She frequently finds that estrogen replacement or birth-control pills ease some memory problems.
Researchers at University of California-Los Angeles, analyzing data from the multi-center Study of Women's Health Across the Nation, found that two-thirds of 2,300 women aged 45 to 57 experienced some memory problems during their menopausal transition time, and that those who began hormone-replacement therapy before their last period had more improvement than those who waited.
Birth-control pills or patches can alleviate perimenopausal symptoms by putting a woman's natural ovarian function at rest and providing a steady level of hormones that keep brain function on an even keel. They also prevent pregnancy, which can be more problematic with other methods when periods have become unpredictable.
The pill does have some downsides—including a slightly higher risk of breast cancer in some cases. Women over 35 who smoke or have tested positive for a gene mutation that predisposes them to breast cancer should not take oral contraceptives. But birth-control pills also lower the risk of uterine and ovarian cancer.
Dr. Goldstein notes that today's pills contain only a half to a quarter of the amount of those in decades past, and because they suppress natural ovulation, women are exposed to lower amounts of estrogen while on a low-dose pill than they would if they were ovulating normally.
He often suggests that women who are encountering symptoms try going on birth-control pills for two months to see how they feel. "I've seen people who have significant improvement. I've seen women who have moderate improvement and women who have no improvement," on the pill, Dr. Goldstein says.
The proportion of hormones varies in pills. Dr. Vliet notes that if women taking a very-low estrogen pill still have headaches, depression, low libido or fatigue, they should ask about switching to a brand with more estrogen and less progestin, such as Femcon, Ovcon 35, Ortho-Cyclen or Yasmin.
A more complicated option is to give perimenopausal women estrogen and progesterone to augment the decline in what their ovaries are making—ideally using an estradial patch or gel and a natural progesterone.
Some doctors, like Dr. Vliet, believe in checking specific hormone levels with blood tests; others say there are too many variations in blood levels and go by symptoms instead.
For women who don't want to take hormones, a few lifestyle changes can help with perimenopausal symptoms. Exercise helps ward off weight gain and enhance serotonin and endorphins. Eating a balance of proteins and carbohydrates can keep weight and blood sugar on an even keel. Drinking alcohol in moderation can lower the risk of heart disease, but drinking more than one glass a day raises the risk of breast cancer and can interfere with sleep.
"I know women who drink a half a bottle of wine every night to deal with their perimenopause symptoms, but they are raising their risk of breast cancer far more than taking estrogen would," says Dr. Vliet.
As for herbal remedies such as black cohosh, wild-yam root and dong quai, the limited studies that have been done have not shown them to have any more benefit than placebos.
If a perimenopausal woman does use birth-control pills, how long should she stay on them? Dr. Goldstein suggests that women stop temporarily when they reach age 51 to see if their natural periods return. If so, he suggests that women return to the pill and try stopping again in six months.
Going off birth-control pills at menopause can bring on the very symptoms they were preventing earlier. In many women, those symptoms will subside. "In a way, you can compare it to puberty—a lot of people have irregular cycles then, and they are more moody, but it generally goes away in a few years," says Dr. Gass.
If bothersome symptoms persist, women can transition into hormone-replacement therapy, which uses a much lower dose of hormones than birth-control pills. The key is to start early, during or soon after menopause. While the Women's Health Initiative (WHI) study found that hormone therapy raised the risk of cardiovascular disease, stroke and breast cancer, the women studied were generally over 65 years old and 10 years past menopause. Subsequent analyses of the data showed that women who started on hormone therapy within five years of menopause had a protective effect instead.
Marketers of so-called bio-identical hormones made in compounding pharmacies often say their products alleviate many of the cognitive and mood symptoms of perimenopause and are safer than standard prescription forms. Yet many of the same "bio-identical" hormones are available in prescription forms that have been approved and inspected by the Food and Drug Administration, unlike combinations from compounding pharmacies. Indeed, another study presented at the NAMS annual conference in Chicago last week found that hormone products made by compounding pharmacies often contained lower doses of hormones than their labels suggested.
All women experience perimenopause differently. The best first step is to undergo a comprehensive exam from an ob/gyn or primary-care doctor to assess blood pressure, cholesterol, bone density and hormone levels including thyroid, all of which are affected by estrogen. Heavy bleeding in perimenopause can be a symptom of a serious condition, such as uterine cancer.
If doctors still dismiss symptoms as "all in your head," or simple aging, persist or find another doctor. "Trust your symptoms," says Dr. Devi, "and seek help accordingly."
The Birth-Control Riddle
Fifty Years After the Pill's Debut, Almost Half of Pregnancies in the U.S. Are Unplanned
Melinda Beck : WSJ Article : April 20, 2010
Next month marks the 50th anniversary of the birth-control pill in the U.S. The dawn of dependable contraception not only ended the post-war baby boom, it also ignited the sexual revolution and helped millions of women to enter the work force.
Nowadays, women can choose from a bevy of birth-control options, including pills, patches and rings that allow them to have as few periods as they like, even none. Implants and intrauterine devices (IUDs) can prevent pregnancy for years at a time and eliminate the need to refill and remember. Morning-after pills that can decrease the risk from unprotected sex are available without a prescription even to teenagers. Women who want to end their fertility permanently can do so in a doctor's office without undergoing surgery. Abstinence is still taught in many schools and homes as being 100% effective if followed diligently.
Yet despite all these options, the rates of unplanned pregnancies remain high: Almost half of all pregnancies in the U.S.—some 3.1 million a year—are unintended, according to the most recent government survey, from 2001. One out of every two American women aged 15 to 44 has at least one unplanned pregnancy in her lifetime. Among unmarried women in their 20s, seven out of 10 pregnancies are unplanned.
An updated version of those numbers from the 2006 National Survey of Family Growth is expected to be released next month. But population experts don't anticipate much change; the rate of unplanned pregnancy was the same in 1994, and smaller studies have found that even newer birth-control methods haven't made much of a dent.
Why are the numbers so high?
The answer is a complex tangle of cultural, religious, behavioral, educational and economic factors. Many of those unplanned pregnancies become wanted babies. About a million are aborted each year and others are miscarried.
Almost half (48%) of unintended pregnancies involve contraceptive failures. In 52% of the cases, couples used no birth control at all. Cost is a factor for some of them. Even though most insurers now cover contraceptives, co-pays and deductibles can still present obstacles.
And many young people are in "the fog zone" in which their beliefs about pregnancy don't match their behaviors, according to a 2009 report by the National Campaign to End Teen and Unplanned Pregnancy. In a survey conducted by the Guttmacher Institute of 1,800 single men and women aged 18 to 29, more than 80% of both sexes said it was important to them to avoid pregnancy right now, yet 43% of those who are sexually active said they used no contraception or used it inconsistently.
Some population experts say the rates of unintended pregnancy would be far lower if more women used IUDs and implants that prevent pregnancy for years at a time. Only about 3% of American women currently do.
"There are terrible misperceptions about these methods— and about all forms of contraception," says James Trussell, director of the Office of Population Research at Princeton University.
Many traditional forms of contraception have been updated in recent years. Here's a look at the latest developments:
The New IUDs:
The IUD got a bad name in the 1970s due to the Dalkon Shield, whose design turned out to make it easy for bacteria and STDs from the vagina to ascend into the uterus and fallopian tubes, causing pelvic-inflammatory disease (PID) and infertility. After hundreds of lawsuits and several deaths, the shield was discontinued in 1974, and doctors were urged to remove them from women in 1980.
IUDs available in the U.S. now are much safer. The ParaGard is made with copper that is toxic to sperm, lasts up to 12 years and doesn't affect a woman's hormone levels. Mirena releases a small amount of progestin that blocks ovulation, and lasts up to five years. Both are more than 99% effective at preventing pregnancy.
Jeffrey Peipert, a top researcher at Washington University in St. Louis, notes that about 18% of female ob/gyns have opted for IUDs. "The most educated consumers are using the most effective methods at high rates," he says.
Cost: $175 to $700 for years of protection.
Downsides: IUDs do not protect against STDs. The ParaGard may increase cramping. In rare cases, IUDs can slip out or push through the uterine lining.
The Implant:
Another long-acting contraceptive is Implanon, a rod a doctor inserts in a woman's arm. It prevents pregnancy for up to three years.
A previous implant called Norplant was beset by lawsuits over side effects and scarring and withdrawn from the U.S. market in 2002.
Doctors are required to undergo special training to insert and remove Implanon, which is also 99% effective at preventing pregnancy.
Cost: $400 to $800 for up to three years' protection.
Downsides: Irregular bleeding, possible headaches, sore breasts, nausea, pain at the insertion site. No STD protection.
Hormone Pills, Patches And Rings:
The first birth-control pills had very high doses of hormones, and side effects including mood swings, weight gain and blood clots were common.
Dosages have since dropped to about one-tenth of their original strength. There are far fewer side effects and a host of benefits, including regularized periods and reduced acne, bloating, premenstrual syndrome and less bleeding due to uterine fibroids. What's more, the longer a woman uses the pill, the lower her risk of ovarian and endometrial cancer.
Still, some women remain wary. "For some reason, it has a really bad rap," says Rachel Bernstein, an ob/gyn in Fort Lauderdale, Fla., who says she has to explain that these days, the benefits outweigh the risks.
Today, there are about 40 different brands of birth-control pills, most of which contain a combination of estrogen and progestin.
Other versions work the same way, including the NuvaRing, a flexible ring that is inserted in the vagina every month and worn for three weeks, then removed for one week, and the Ortho-Evra patch, which is replaced every week for three weeks, followed by a patch-free week.
Newer pills, like Seasonique, allow women to have only four periods a year; Lybrel, approved in 2007, is designed to be taken for 365 days straight with no period. Experts say there is no health reason that women need to have a period if they are not ovulating or building up uterine lining each month. The traditional pills were designed to include a monthly period in part so that women would be reassured that they weren't pregnant.
Hormonal birth control is 99% effective at preventing pregnancy if used correctly, but in average use, with some forgetfulness, it's only 92% effective.
Cost: $15 to $50 per month
Downsides: Women who have breast cancer should not take estrogen, but the pill does not seem to increase the risk of getting it. It can cause blood clots in rare cases. But, says Vanessa Cullins, vice president for medical affairs of Planned Parenthood Federation: "We have to keep these things in perspective. A woman's risk for those problems is substantially higher during pregnancy."
A more long-lasting form, the Depo-Provera injection, releases progestin only and prevents pregnancy for 12 weeks. It can be used by women who can't take estrogen and by those who are breast-feeding.
Cost: Each injection costs $35 to $75
Downsides: Possible weight gain, depression, blood clots and bone thinning; usually recommended only for women who can't use other forms.
Condoms, Caps And Sponges:
Using a receptacle to contain sperm during sex literally goes back to caveman days: It's depicted on the wall of a cave in France from 12,000 B.C. Over the millennia, condoms have been made of paper, animal intestines, leather and linen (a favorite of womanizer Giacomo Casanova in 18th century Italy). Charles Goodyear paved the way for mass production of "rubbers" when he patented the vulcanization of rubber in 1843, and they were a mainstay of birth-control efforts until the pill emerged as more dependable and convenient.
The popularity of condoms soared in the 1980s, when the AIDS epidemic alerted public-health officials to the need for protection against STDs as well as pregnancy. Nowadays, condoms come in a variety of sizes, colors, materials and even flavors. Most are made of latex, but polyurethane and lamb-skin condoms are also available.
Cost: generally $1 each, but sometimes free; available without a prescription.
Effectiveness is still an issue. Roughly two of every 100 women whose partners use condoms correctly become pregnant each year, as do 15 of 100 women whose partners don't use them correctly.
Female condoms, plastic pouches inserted in the vagina before intercourse, are less effective. About five in 100 women who always use them correctly become pregnant each year, as do 21 out of 100 women who don't always use them correctly. They cost about $4 each and are sold without a prescription.
Other "barrier" birth-control methods include the Today Sponge, an updated version of the brand made famous on "Seinfield," that involves a spermicide-containing foam disc that covers the cervix and blocks sperm from entering; the FemCap, a reusable silicon cup filled with spermicide that covers the cervix, and the diaphragm, a much older rubber bowl filled with spermicide. Although all three are inexpensive, don't involve hormones and have few side effects, they are inconvenient, don't protect against STDs and are not very effective. Even with careful use, as many as 20 out of 100 women using them each become pregnant.
Even combined, they make up a very small percentage of birth-control users today. "There's a whole generation of women who didn't pick them up," says Mr. Trussell.
Emergency Contraception:
Versions of "the morning-after pill"—a combination of hormones that abruptly halts ovulation—have been around quietly since the 1970s. In 1999, the FDA approved Plan B, a progestin-only pill that does the same, preventing pregnancy up to five days after unprotected sex or in case of a condom break or forgotten pill. It's currently available in three brands, Plan B, Plan B One-Step and Next Choice. Plan B and Next Choice are 89% effective if used within 72 hours after intercourse.
But emergency contraception is only effective if it is used every time after unprotected sex. "If you don't use it every time, then you are eventually going to get pregnant," says Mr. Trussell, who runs a Web site called Not-2-late.com with information on emergency contraceptives.
Cost: From $10 to $70, without a prescription for teens 17 and older
Downsides: They may cause temporary nausea, headaches and irregular bleeding; no STD protection.
Permanent Birth Control:
Every year, about 700,000 U.S. women who no longer want children have a tubal ligation, a surgical procedure in which the fallopian tubes are cut and tied off. It's an outpatient procedure, but still requires a hospital stay and a cost of $5,000 or more.
A less-invasive version called Essure can be performed in a doctor's office. Using a hysteroscope, the doctor inserts a tiny titanium coil into each tube via the uterus. The woman's own tissue then grows around the coils, forming a natural but permanent barrier.
It's caught on slowly in the U.S., in part because doctors require additional training. Women must also have a follow-up test in three months to make sure a seal has formed, and to use other birth control in the meantime. But it's over 99% effective, without surgery or hormones.
A competing version, called Adiana that uses a smaller implant and radio-frequency energy, was approved by the FDA last year.
Cost: about $2,000
Downsides: The procedure may cause mild cramping, does not protect against STDs and is not reversible.
Vasectomy Variations:
On the male side, there are new variations as well. Traditionally, a doctor makes an incision in the scrotum and cuts the two vas deferens, the tubes that carry sperm out of the testicles, and ties, stitches or seals them closed. The procedure takes about 30 minutes and is done in a doctor's office or clinic.
In one newer version, the doctor pokes a clamp through the skin instead of using a scalpel; there's less bleeding and fewer complications. In another, the vas deferens are locked closed with a clip, though some studies have shown that may not be as effective as other methods.
In general, vasectomies are nearly 100% effective—but since some sperm may remain in the blocked tubes, couples must use another form of birth control for three months. In very rare cases, the tubes may grow back together and permit the passage of sperm again.
Cost: $350 to $1000, covered by insurers and Medicaid.
Downsides: There's some minor pain, swelling and numbness for several days after the procedure, but most men can resume sexual activities within a week. Vasectomies theoretically can be reversed, but they are often not effective. Men should consider them permanent.
Fifty Years After the Pill's Debut, Almost Half of Pregnancies in the U.S. Are Unplanned
Melinda Beck : WSJ Article : April 20, 2010
Next month marks the 50th anniversary of the birth-control pill in the U.S. The dawn of dependable contraception not only ended the post-war baby boom, it also ignited the sexual revolution and helped millions of women to enter the work force.
Nowadays, women can choose from a bevy of birth-control options, including pills, patches and rings that allow them to have as few periods as they like, even none. Implants and intrauterine devices (IUDs) can prevent pregnancy for years at a time and eliminate the need to refill and remember. Morning-after pills that can decrease the risk from unprotected sex are available without a prescription even to teenagers. Women who want to end their fertility permanently can do so in a doctor's office without undergoing surgery. Abstinence is still taught in many schools and homes as being 100% effective if followed diligently.
Yet despite all these options, the rates of unplanned pregnancies remain high: Almost half of all pregnancies in the U.S.—some 3.1 million a year—are unintended, according to the most recent government survey, from 2001. One out of every two American women aged 15 to 44 has at least one unplanned pregnancy in her lifetime. Among unmarried women in their 20s, seven out of 10 pregnancies are unplanned.
An updated version of those numbers from the 2006 National Survey of Family Growth is expected to be released next month. But population experts don't anticipate much change; the rate of unplanned pregnancy was the same in 1994, and smaller studies have found that even newer birth-control methods haven't made much of a dent.
Why are the numbers so high?
The answer is a complex tangle of cultural, religious, behavioral, educational and economic factors. Many of those unplanned pregnancies become wanted babies. About a million are aborted each year and others are miscarried.
Almost half (48%) of unintended pregnancies involve contraceptive failures. In 52% of the cases, couples used no birth control at all. Cost is a factor for some of them. Even though most insurers now cover contraceptives, co-pays and deductibles can still present obstacles.
And many young people are in "the fog zone" in which their beliefs about pregnancy don't match their behaviors, according to a 2009 report by the National Campaign to End Teen and Unplanned Pregnancy. In a survey conducted by the Guttmacher Institute of 1,800 single men and women aged 18 to 29, more than 80% of both sexes said it was important to them to avoid pregnancy right now, yet 43% of those who are sexually active said they used no contraception or used it inconsistently.
Some population experts say the rates of unintended pregnancy would be far lower if more women used IUDs and implants that prevent pregnancy for years at a time. Only about 3% of American women currently do.
"There are terrible misperceptions about these methods— and about all forms of contraception," says James Trussell, director of the Office of Population Research at Princeton University.
Many traditional forms of contraception have been updated in recent years. Here's a look at the latest developments:
The New IUDs:
The IUD got a bad name in the 1970s due to the Dalkon Shield, whose design turned out to make it easy for bacteria and STDs from the vagina to ascend into the uterus and fallopian tubes, causing pelvic-inflammatory disease (PID) and infertility. After hundreds of lawsuits and several deaths, the shield was discontinued in 1974, and doctors were urged to remove them from women in 1980.
IUDs available in the U.S. now are much safer. The ParaGard is made with copper that is toxic to sperm, lasts up to 12 years and doesn't affect a woman's hormone levels. Mirena releases a small amount of progestin that blocks ovulation, and lasts up to five years. Both are more than 99% effective at preventing pregnancy.
Jeffrey Peipert, a top researcher at Washington University in St. Louis, notes that about 18% of female ob/gyns have opted for IUDs. "The most educated consumers are using the most effective methods at high rates," he says.
Cost: $175 to $700 for years of protection.
Downsides: IUDs do not protect against STDs. The ParaGard may increase cramping. In rare cases, IUDs can slip out or push through the uterine lining.
The Implant:
Another long-acting contraceptive is Implanon, a rod a doctor inserts in a woman's arm. It prevents pregnancy for up to three years.
A previous implant called Norplant was beset by lawsuits over side effects and scarring and withdrawn from the U.S. market in 2002.
Doctors are required to undergo special training to insert and remove Implanon, which is also 99% effective at preventing pregnancy.
Cost: $400 to $800 for up to three years' protection.
Downsides: Irregular bleeding, possible headaches, sore breasts, nausea, pain at the insertion site. No STD protection.
Hormone Pills, Patches And Rings:
The first birth-control pills had very high doses of hormones, and side effects including mood swings, weight gain and blood clots were common.
Dosages have since dropped to about one-tenth of their original strength. There are far fewer side effects and a host of benefits, including regularized periods and reduced acne, bloating, premenstrual syndrome and less bleeding due to uterine fibroids. What's more, the longer a woman uses the pill, the lower her risk of ovarian and endometrial cancer.
Still, some women remain wary. "For some reason, it has a really bad rap," says Rachel Bernstein, an ob/gyn in Fort Lauderdale, Fla., who says she has to explain that these days, the benefits outweigh the risks.
Today, there are about 40 different brands of birth-control pills, most of which contain a combination of estrogen and progestin.
Other versions work the same way, including the NuvaRing, a flexible ring that is inserted in the vagina every month and worn for three weeks, then removed for one week, and the Ortho-Evra patch, which is replaced every week for three weeks, followed by a patch-free week.
Newer pills, like Seasonique, allow women to have only four periods a year; Lybrel, approved in 2007, is designed to be taken for 365 days straight with no period. Experts say there is no health reason that women need to have a period if they are not ovulating or building up uterine lining each month. The traditional pills were designed to include a monthly period in part so that women would be reassured that they weren't pregnant.
Hormonal birth control is 99% effective at preventing pregnancy if used correctly, but in average use, with some forgetfulness, it's only 92% effective.
Cost: $15 to $50 per month
Downsides: Women who have breast cancer should not take estrogen, but the pill does not seem to increase the risk of getting it. It can cause blood clots in rare cases. But, says Vanessa Cullins, vice president for medical affairs of Planned Parenthood Federation: "We have to keep these things in perspective. A woman's risk for those problems is substantially higher during pregnancy."
A more long-lasting form, the Depo-Provera injection, releases progestin only and prevents pregnancy for 12 weeks. It can be used by women who can't take estrogen and by those who are breast-feeding.
Cost: Each injection costs $35 to $75
Downsides: Possible weight gain, depression, blood clots and bone thinning; usually recommended only for women who can't use other forms.
Condoms, Caps And Sponges:
Using a receptacle to contain sperm during sex literally goes back to caveman days: It's depicted on the wall of a cave in France from 12,000 B.C. Over the millennia, condoms have been made of paper, animal intestines, leather and linen (a favorite of womanizer Giacomo Casanova in 18th century Italy). Charles Goodyear paved the way for mass production of "rubbers" when he patented the vulcanization of rubber in 1843, and they were a mainstay of birth-control efforts until the pill emerged as more dependable and convenient.
The popularity of condoms soared in the 1980s, when the AIDS epidemic alerted public-health officials to the need for protection against STDs as well as pregnancy. Nowadays, condoms come in a variety of sizes, colors, materials and even flavors. Most are made of latex, but polyurethane and lamb-skin condoms are also available.
Cost: generally $1 each, but sometimes free; available without a prescription.
Effectiveness is still an issue. Roughly two of every 100 women whose partners use condoms correctly become pregnant each year, as do 15 of 100 women whose partners don't use them correctly.
Female condoms, plastic pouches inserted in the vagina before intercourse, are less effective. About five in 100 women who always use them correctly become pregnant each year, as do 21 out of 100 women who don't always use them correctly. They cost about $4 each and are sold without a prescription.
Other "barrier" birth-control methods include the Today Sponge, an updated version of the brand made famous on "Seinfield," that involves a spermicide-containing foam disc that covers the cervix and blocks sperm from entering; the FemCap, a reusable silicon cup filled with spermicide that covers the cervix, and the diaphragm, a much older rubber bowl filled with spermicide. Although all three are inexpensive, don't involve hormones and have few side effects, they are inconvenient, don't protect against STDs and are not very effective. Even with careful use, as many as 20 out of 100 women using them each become pregnant.
Even combined, they make up a very small percentage of birth-control users today. "There's a whole generation of women who didn't pick them up," says Mr. Trussell.
Emergency Contraception:
Versions of "the morning-after pill"—a combination of hormones that abruptly halts ovulation—have been around quietly since the 1970s. In 1999, the FDA approved Plan B, a progestin-only pill that does the same, preventing pregnancy up to five days after unprotected sex or in case of a condom break or forgotten pill. It's currently available in three brands, Plan B, Plan B One-Step and Next Choice. Plan B and Next Choice are 89% effective if used within 72 hours after intercourse.
But emergency contraception is only effective if it is used every time after unprotected sex. "If you don't use it every time, then you are eventually going to get pregnant," says Mr. Trussell, who runs a Web site called Not-2-late.com with information on emergency contraceptives.
Cost: From $10 to $70, without a prescription for teens 17 and older
Downsides: They may cause temporary nausea, headaches and irregular bleeding; no STD protection.
Permanent Birth Control:
Every year, about 700,000 U.S. women who no longer want children have a tubal ligation, a surgical procedure in which the fallopian tubes are cut and tied off. It's an outpatient procedure, but still requires a hospital stay and a cost of $5,000 or more.
A less-invasive version called Essure can be performed in a doctor's office. Using a hysteroscope, the doctor inserts a tiny titanium coil into each tube via the uterus. The woman's own tissue then grows around the coils, forming a natural but permanent barrier.
It's caught on slowly in the U.S., in part because doctors require additional training. Women must also have a follow-up test in three months to make sure a seal has formed, and to use other birth control in the meantime. But it's over 99% effective, without surgery or hormones.
A competing version, called Adiana that uses a smaller implant and radio-frequency energy, was approved by the FDA last year.
Cost: about $2,000
Downsides: The procedure may cause mild cramping, does not protect against STDs and is not reversible.
Vasectomy Variations:
On the male side, there are new variations as well. Traditionally, a doctor makes an incision in the scrotum and cuts the two vas deferens, the tubes that carry sperm out of the testicles, and ties, stitches or seals them closed. The procedure takes about 30 minutes and is done in a doctor's office or clinic.
In one newer version, the doctor pokes a clamp through the skin instead of using a scalpel; there's less bleeding and fewer complications. In another, the vas deferens are locked closed with a clip, though some studies have shown that may not be as effective as other methods.
In general, vasectomies are nearly 100% effective—but since some sperm may remain in the blocked tubes, couples must use another form of birth control for three months. In very rare cases, the tubes may grow back together and permit the passage of sperm again.
Cost: $350 to $1000, covered by insurers and Medicaid.
Downsides: There's some minor pain, swelling and numbness for several days after the procedure, but most men can resume sexual activities within a week. Vasectomies theoretically can be reversed, but they are often not effective. Men should consider them permanent.